Novavax COVID-19 Vaccine Gets FDA Nod, Under Strict Guidelines
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Gets FDA Nod, Under Strict Guidelines
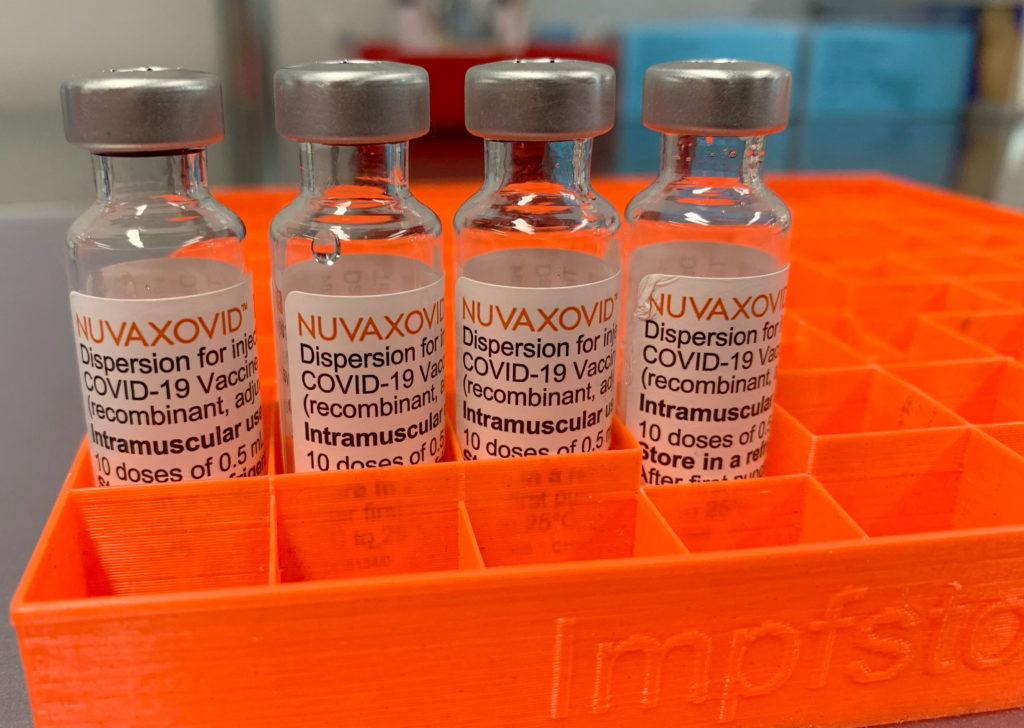
The FDA has finally approved the Novavax COVID-19 vaccine, Nuvaxovid, offering a new option for Americans, but with specific limitations. This protein-subunit vaccine, a different technology than the mRNA vaccines from Pfizer-BioNTech and Moderna, has been granted Emergency Use Authorization (EUA), but its rollout will be carefully managed. This approval marks a significant development in the fight against the COVID-19 pandemic, offering a potential solution for individuals hesitant about mRNA vaccines.
This long-awaited approval comes after months of review and analysis by the Food and Drug Administration (FDA). The authorization is conditional, meaning the FDA will continue monitoring its safety and effectiveness. The decision follows a rigorous evaluation process, addressing concerns raised about the vaccine's efficacy and safety profile.
How Does the Novavax Vaccine Differ?
Unlike the mRNA vaccines that utilize messenger RNA to instruct cells to produce a viral protein, Novavax's Nuvaxovid is a protein-subunit vaccine. This technology uses purified fragments of the SARS-CoV-2 virus to trigger an immune response. This approach has been used for years in other vaccines, potentially making it more appealing to vaccine-hesitant individuals who may have concerns about the newer mRNA technology. This difference in technology could expand vaccine access for a segment of the population.
Strict Guidelines and Limited Rollout
The FDA's approval isn't without conditions. The rollout of the Novavax vaccine will be closely monitored, and the agency will continue to gather data on its safety and effectiveness in real-world settings. This cautious approach reflects the FDA's commitment to ensuring the safety and efficacy of all vaccines authorized for use in the United States.
Key limitations and considerations:
- Limited supply: Initially, the availability of the Novavax vaccine will be restricted, potentially leading to limited access in certain areas.
- Specific age groups: While the EUA covers adults 18 years and older, further studies may expand its use to other age groups.
- Ongoing monitoring: The FDA will continue to monitor the vaccine's safety and efficacy, conducting post-market surveillance to detect any potential adverse effects.
- Booster shots: The need for booster doses with the Novavax vaccine is currently under investigation.
What Does this Mean for the Future of COVID-19 Vaccination?
The approval of the Novavax COVID-19 vaccine represents a significant milestone. It provides an additional vaccine option for individuals who might have preferred a protein-subunit approach. However, it’s crucial to remember that all currently available COVID-19 vaccines remain important tools in protecting against severe illness, hospitalization, and death. The addition of Novavax's vaccine to the arsenal strengthens the overall strategy in combating the virus. This increased vaccine choice aims to improve vaccination rates and contribute to broader community immunity.
Finding the Novavax Vaccine
For information on where to find the Novavax COVID-19 vaccine in your area, you should contact your local health department or primary care physician. You can also check the for updates on vaccine availability and distribution.
Disclaimer: This article provides information for general knowledge and informational purposes only, and does not constitute medical advice. Consult with a healthcare professional for any health concerns or before making any decisions related to your health or treatment.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, Under Strict Guidelines. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
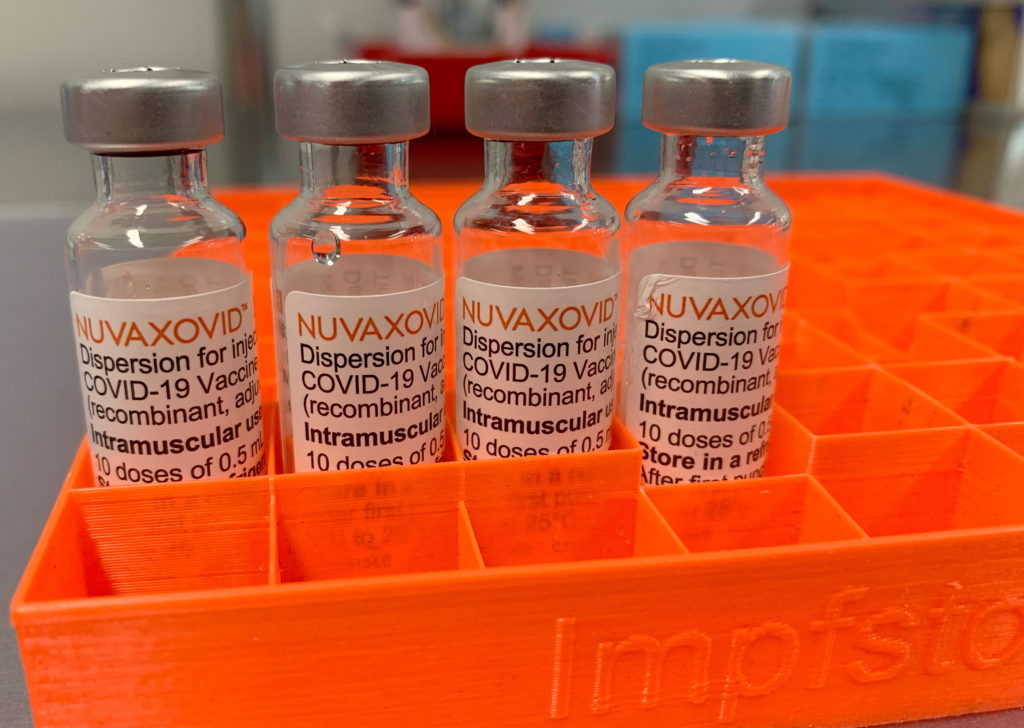 Novavax Covid 19 Vaccine Fda Approval And Its Specific Usage Guidelines
May 21, 2025
Novavax Covid 19 Vaccine Fda Approval And Its Specific Usage Guidelines
May 21, 2025 -
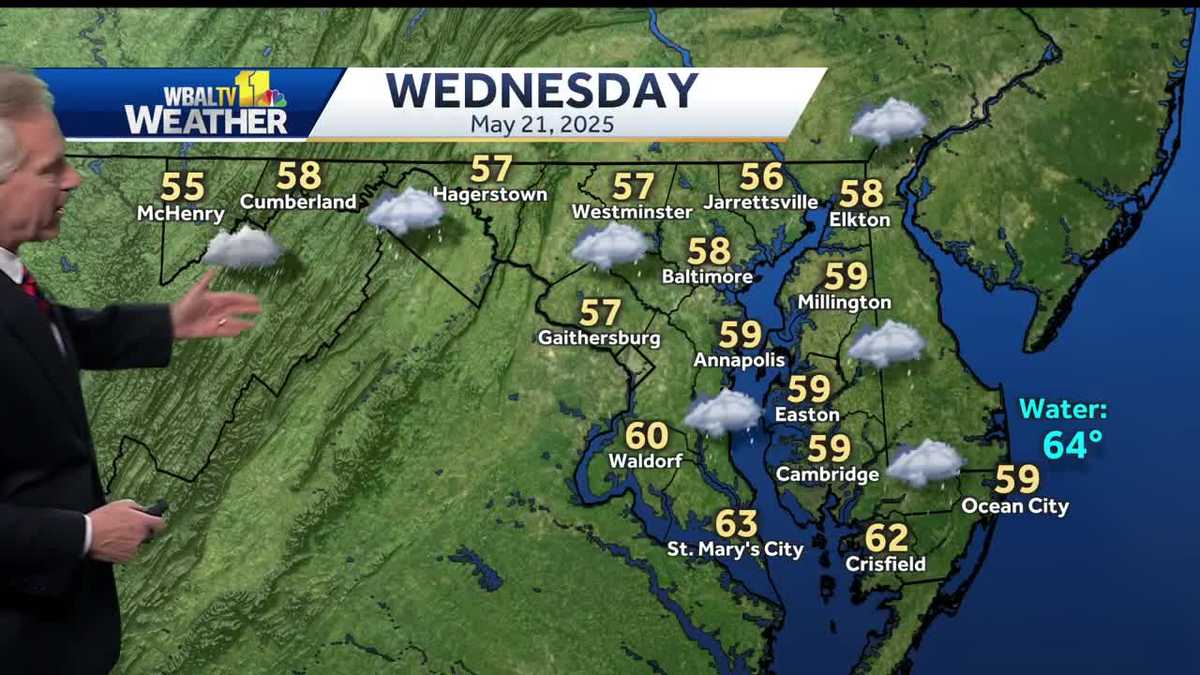 Region Braces For Rain And Chilly Temperatures On Wednesday
May 21, 2025
Region Braces For Rain And Chilly Temperatures On Wednesday
May 21, 2025 -
 League Of Legends 2025 Hall Of Famer Revealed Pricing Concerns For New Skin
May 21, 2025
League Of Legends 2025 Hall Of Famer Revealed Pricing Concerns For New Skin
May 21, 2025 -
 Church Defiled Santa Rosa Police Respond To Teenagers Act Of Vandalism
May 21, 2025
Church Defiled Santa Rosa Police Respond To Teenagers Act Of Vandalism
May 21, 2025 -
 Juvenile Delinquency Church Break In And Vandalism Case
May 21, 2025
Juvenile Delinquency Church Break In And Vandalism Case
May 21, 2025
