Novavax COVID-19 Vaccine: FDA Approval And Its Specific Usage Guidelines
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
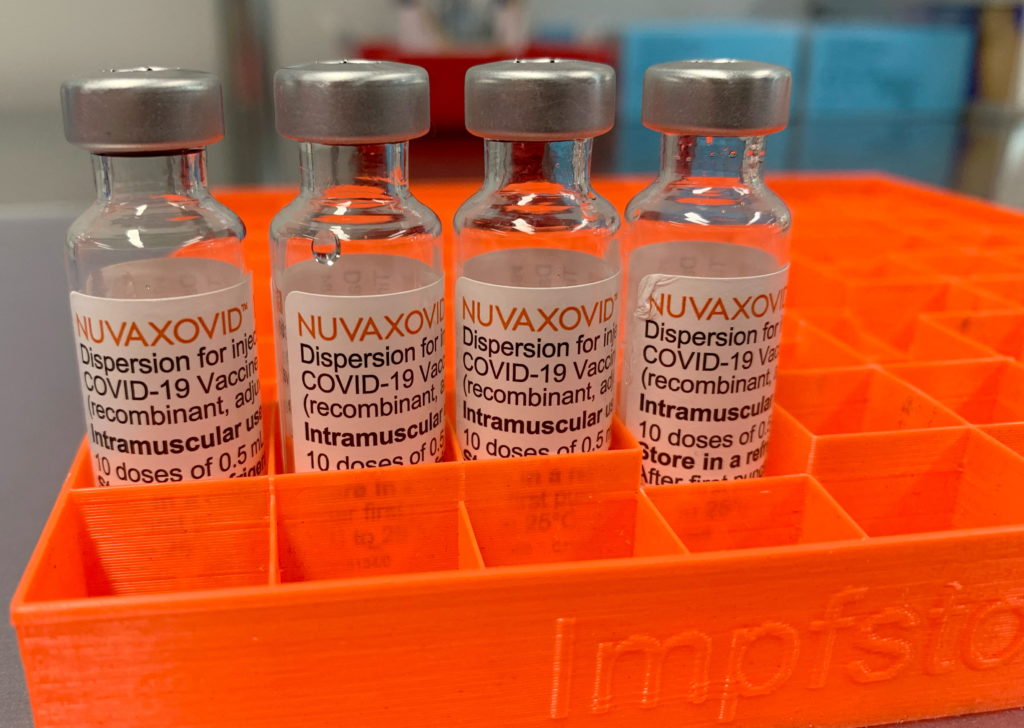
Novavax COVID-19 Vaccine: FDA Approval, Usage, and What You Need to Know
The Novavax COVID-19 vaccine, Nuvaxovid, represents a significant addition to the arsenal of tools combating the pandemic. Its arrival, following FDA approval, offers a different approach to vaccination, providing an alternative for those who may have hesitated due to concerns about mRNA technology used in other vaccines. But what exactly does FDA approval mean, and what are the specific usage guidelines surrounding this protein-subunit vaccine? Let's delve into the details.
FDA Approval and What it Means
The FDA's approval of Nuvaxovid signifies a rigorous review process confirming its safety and efficacy. Unlike Emergency Use Authorization (EUA), which allows for quicker access during public health emergencies, FDA approval requires a more comprehensive evaluation of clinical trial data, demonstrating the vaccine's effectiveness in preventing COVID-19. This approval solidifies its place as a reliable and safe vaccination option. This process involved detailed analysis of the vaccine's ability to prevent severe illness, hospitalization, and death. The rigorous standards ensure public trust and confidence in the vaccine's safety and effectiveness.
Understanding the Novavax Vaccine: A Protein-Subunit Approach
Unlike mRNA vaccines which utilize messenger RNA to instruct cells to produce viral proteins, Novavax employs a protein-subunit approach. This means the vaccine contains purified fragments of the SARS-CoV-2 virus's spike protein. This protein is what the virus uses to enter human cells. By introducing this protein, the body creates an immune response, equipping it to defend against future infections. This method is familiar to many, as it’s similar to how many traditional vaccines, like the Hepatitis B vaccine, operate.
Specific Usage Guidelines and Recommendations:
- Dosage: The Novavax COVID-19 vaccine requires a two-dose primary series, administered three to eight weeks apart.
- Target Population: Currently, the vaccine is approved for individuals 18 years of age and older. Further studies may expand this age range in the future.
- Side Effects: As with other vaccines, some individuals may experience common side effects such as injection site pain, fatigue, headache, muscle aches, and fever. These side effects are usually mild and temporary. [Link to CDC website on vaccine side effects]
- Contraindications: Individuals with a known history of severe allergic reactions to any component of the vaccine should not receive it. Consult your physician if you have any concerns or pre-existing conditions.
- Booster Shots: Information regarding booster shots will be updated by the CDC and FDA as data becomes available. [Link to CDC website on COVID-19 vaccine boosters]
Novavax Vaccine vs. Other COVID-19 Vaccines:
While offering a different mechanism of action, the Novavax vaccine shares the common goal of reducing COVID-19's severity. Clinical trials demonstrated its effectiveness in preventing symptomatic infection. The availability of multiple vaccine types allows individuals to choose the vaccine that best suits their individual needs and preferences, after consultation with their healthcare provider.
Conclusion:
The FDA approval of the Novavax COVID-19 vaccine provides a valuable alternative within the broader vaccination strategy. Its protein-subunit technology may appeal to those seeking a different approach, contributing to a more comprehensive and accessible vaccination program. Always consult with your healthcare provider to determine the best vaccination strategy for you. Staying informed about the latest updates on vaccine safety and efficacy is vital in combating the ongoing pandemic.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, protein-subunit vaccine, vaccination, COVID-19, vaccine safety, vaccine efficacy, usage guidelines, side effects, booster shot, mRNA vaccine.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Approval And Its Specific Usage Guidelines. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Assassins Creed Shadows Why Ubisoft Forbids Killing Animals
May 21, 2025
Assassins Creed Shadows Why Ubisoft Forbids Killing Animals
May 21, 2025 -
 Santa Rosa Church Faces Desecration Two Teenagers Charged With Public Indecency
May 21, 2025
Santa Rosa Church Faces Desecration Two Teenagers Charged With Public Indecency
May 21, 2025 -
 The Power Of Research Strengthening America Through Medical And Scientific Discovery
May 21, 2025
The Power Of Research Strengthening America Through Medical And Scientific Discovery
May 21, 2025 -
 Russia Intensifies Ukraine Assault Trump Seeks Cease Fire Through Talks With Putin And Zelensky
May 21, 2025
Russia Intensifies Ukraine Assault Trump Seeks Cease Fire Through Talks With Putin And Zelensky
May 21, 2025 -
 Heartfelt Welcome Ellen De Generes Return To Social Media
May 21, 2025
Heartfelt Welcome Ellen De Generes Return To Social Media
May 21, 2025
