Novavax COVID-19 Vaccine Gets FDA Nod, Specific Use Restrictions Apply
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Gets FDA Nod, But with Specific Use Restrictions
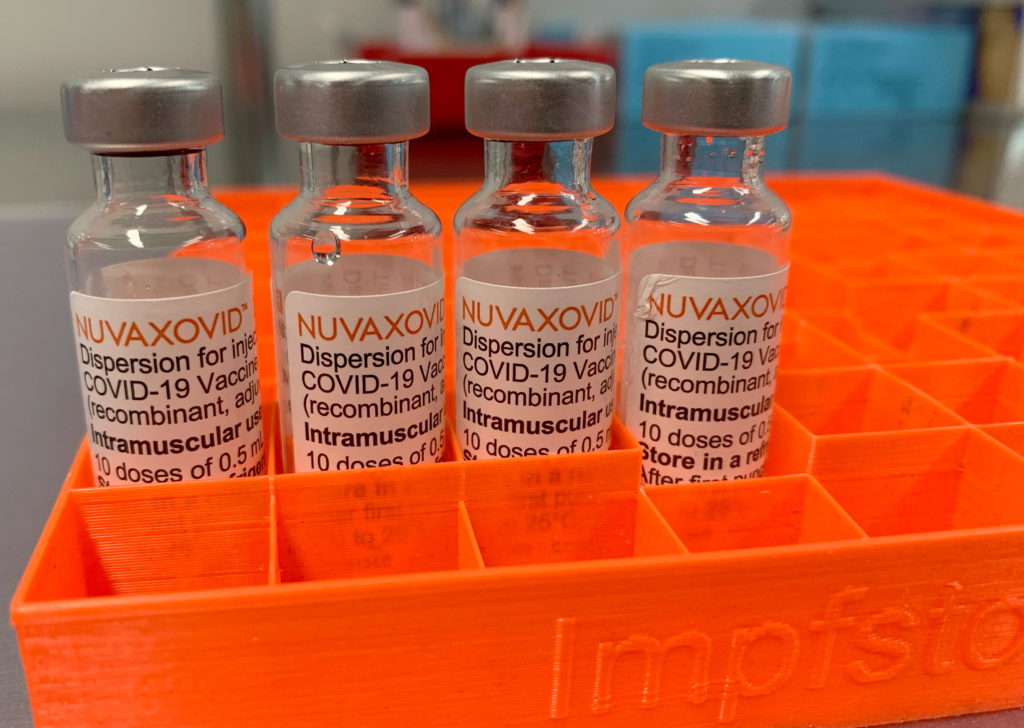
Novavax's COVID-19 vaccine, Nuvaxovid, has finally received full approval from the FDA, marking a significant milestone in the fight against the pandemic. However, the approval comes with specific use restrictions, limiting its immediate impact.
The U.S. Food and Drug Administration (FDA) granted full approval to Novavax's COVID-19 vaccine, Nuvaxovid, on August 1, 2023, for individuals 18 years of age and older. This marks a significant shift from its previous Emergency Use Authorization (EUA), offering a new option for those seeking a protein-subunit vaccine. However, the approval isn't without caveats. The FDA's decision highlights the complexities of vaccine deployment and the ongoing need for tailored approaches to public health challenges.
Understanding Nuvaxovid's Approval and Restrictions
Unlike mRNA vaccines like Pfizer-BioNTech and Moderna, Nuvaxovid uses a more traditional protein-subunit technology. This technology involves using harmless pieces of the virus to trigger an immune response. Many individuals have expressed a preference for this type of vaccine due to its established safety profile and perceived lower risk of side effects. This approval could therefore cater to a segment of the population hesitant to receive mRNA vaccines.
However, the FDA's approval is not a blanket endorsement for widespread use. The agency's decision emphasizes the importance of carefully considering the individual's medical history and available alternatives. The restricted use stems from several factors:
- Limited Efficacy Data: While effective, Nuvaxovid's efficacy data against newer variants might not be as robust as some other authorized vaccines. Further studies are ongoing to assess its long-term efficacy and protection against emerging strains.
- Competition in the Market: The current landscape features high vaccination rates in many regions, and other vaccines have already established their safety and efficacy profiles. This reduces the immediate urgency for a new vaccine entry.
- Manufacturing Capacity: The production capacity for Nuvaxovid may currently limit the scale of its immediate deployment.
Who Might Benefit from Nuvaxovid?
Despite the restrictions, certain populations could still significantly benefit from the availability of Nuvaxovid:
- Individuals hesitant about mRNA vaccines: The protein-subunit technology may appeal to those with concerns about mRNA vaccines. This approval offers a valuable alternative within a diversified vaccine portfolio.
- Specific medical needs: In certain cases, a physician might recommend Nuvaxovid based on a patient's specific medical history or pre-existing conditions.
- Future variant adaptation: The platform's adaptability could make it quicker to adapt to future COVID-19 variants, although this remains subject to further research and development.
What This Means for the Future of COVID-19 Vaccination
The full approval of Nuvaxovid represents a positive development for the ongoing global fight against COVID-19. It offers an alternative vaccine technology and expands options for vaccination strategies. However, the specific use restrictions underscore the evolving nature of the pandemic and the need for continued vigilance in vaccine development and deployment. Future research will be key to determining the long-term role of Nuvaxovid in pandemic preparedness and response.
This is a developing story. For the latest information on COVID-19 vaccination, please consult your healthcare provider and refer to official sources like the CDC and FDA websites. [Link to CDC website] [Link to FDA website]

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, Specific Use Restrictions Apply. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Record Bitcoin Etf Investments Understanding The Current Market Trend
May 20, 2025
Record Bitcoin Etf Investments Understanding The Current Market Trend
May 20, 2025 -
 Reality Tv And Immigration A Dhs Proposal Sparks Debate
May 20, 2025
Reality Tv And Immigration A Dhs Proposal Sparks Debate
May 20, 2025 -
 A New Era For Peaky Blinders Creator Confirms Next Series
May 20, 2025
A New Era For Peaky Blinders Creator Confirms Next Series
May 20, 2025 -
 Balis Plea Global Action Needed For Responsible Tourism
May 20, 2025
Balis Plea Global Action Needed For Responsible Tourism
May 20, 2025 -
 International Support Urged For Balis Tourist Safety Initiatives
May 20, 2025
International Support Urged For Balis Tourist Safety Initiatives
May 20, 2025
