Novavax COVID-19 Vaccine Gets FDA Nod, Significant Usage Restrictions Apply
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
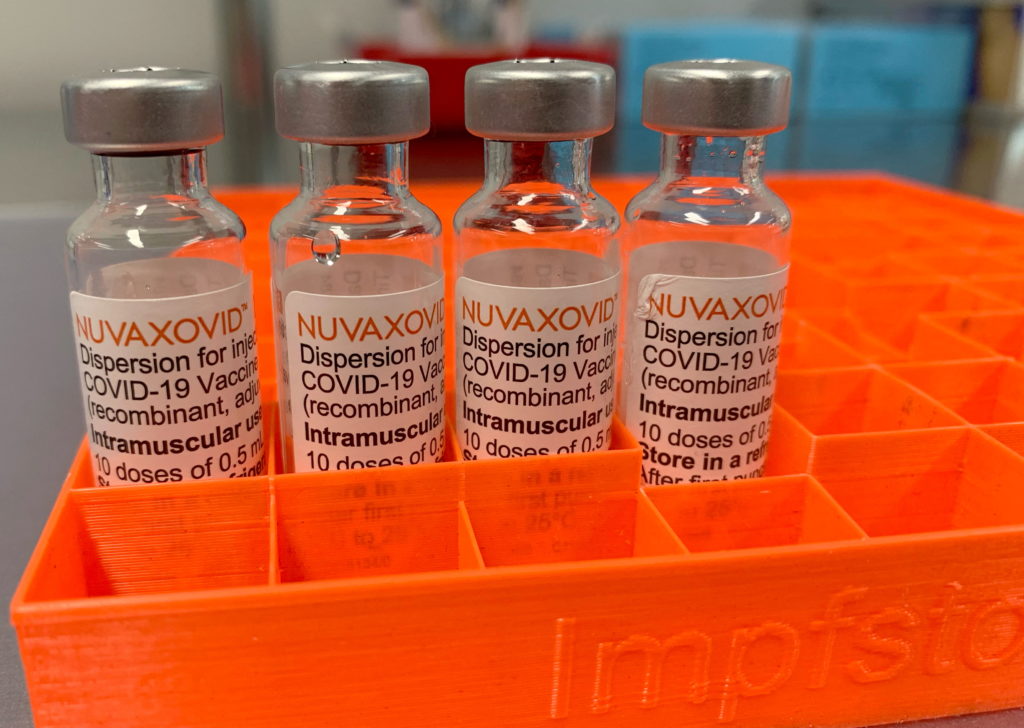
Novavax COVID-19 Vaccine Gets FDA Nod, but with Significant Usage Restrictions
The FDA has finally approved the Novavax COVID-19 vaccine, Nuvaxovid, but its rollout will be limited due to several factors. This long-awaited approval marks a significant development in the fight against the pandemic, offering an alternative for individuals hesitant about mRNA vaccines. However, the FDA's decision comes with considerable caveats, impacting its potential widespread use.
The approval, announced [Insert Date of Announcement], grants emergency use authorization (EUA) for Nuvaxovid, a protein subunit vaccine, for individuals 18 years and older. This differs from the mRNA vaccines produced by Pfizer-BioNTech and Moderna, which utilize a different technology to elicit an immune response. This difference may prove crucial for vaccine hesitancy, providing a familiar protein-based alternative for those who have been reluctant to receive mRNA shots. However, the limited scope of the EUA reveals the complexities surrounding this new addition to the COVID-19 vaccine landscape.
Why the Restrictions?
Several factors contribute to the significant usage restrictions placed on the Novavax vaccine:
- Lower Efficacy: Clinical trials showed Nuvaxovid to have slightly lower efficacy compared to mRNA vaccines, particularly against certain COVID-19 variants. While still effective in preventing severe illness and hospitalization, this lower efficacy rate impacts its overall recommendation.
- Logistical Challenges: The Novavax vaccine requires specific storage and handling conditions, potentially making its distribution more challenging compared to mRNA vaccines. This logistical hurdle could limit its accessibility, especially in areas with limited cold-chain infrastructure.
- Limited Demand: With the widespread availability and high uptake of mRNA vaccines, the demand for a new vaccine might be limited. This, combined with the other restrictions, could affect its overall impact on vaccination rates.
- Potential for Adverse Effects: While generally well-tolerated, like all vaccines, Nuvaxovid carries the potential for side effects. The FDA will continue to monitor its safety profile post-authorization. [Link to FDA safety information page].
Who Should Consider the Novavax Vaccine?
Despite the limitations, the Novavax vaccine may be a suitable option for certain individuals:
- Those hesitant about mRNA vaccines: The protein subunit technology used in Nuvaxovid could be a preferred alternative for individuals with concerns about mRNA vaccines.
- Individuals with specific medical conditions: In consultation with their physician, some individuals with pre-existing conditions might find this vaccine a more appropriate choice.
It is crucial to consult with a healthcare professional to determine if the Novavax vaccine is the right choice for you, considering your individual health circumstances and risk factors.
The Future of Nuvaxovid
The approval of the Novavax vaccine represents a step forward in the global fight against COVID-19. While the usage restrictions currently limit its widespread impact, its potential to address vaccine hesitancy and offer an alternative technology remains significant. Further research and data collection will be crucial to better understand its long-term efficacy and safety profile. The FDA will continue to monitor its use and efficacy closely. The long-term implications for the COVID-19 vaccination strategy remain to be seen, but this approval adds another tool to our arsenal in the ongoing pandemic response.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, protein subunit vaccine, mRNA vaccine, vaccine hesitancy, EUA, emergency use authorization, vaccine efficacy, vaccine safety, COVID-19 pandemic, vaccination, healthcare
Call to Action (subtle): Consult your doctor to discuss which COVID-19 vaccine is best for you.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, Significant Usage Restrictions Apply. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Balis Plea Prioritizing Tourist Safety And Responsible Behavior
May 20, 2025
Balis Plea Prioritizing Tourist Safety And Responsible Behavior
May 20, 2025 -
 The Unexpected Bond Jamie Lee Curtis Speaks Out On Her Relationship With Lindsay Lohan
May 20, 2025
The Unexpected Bond Jamie Lee Curtis Speaks Out On Her Relationship With Lindsay Lohan
May 20, 2025 -
 S And P 500 Dow Nasdaq Rise Market Resilience In The Face Of Moodys Downgrade
May 20, 2025
S And P 500 Dow Nasdaq Rise Market Resilience In The Face Of Moodys Downgrade
May 20, 2025 -
 U S Treasury Yields Dip As Federal Reserve Hints At Single 2025 Rate Decrease
May 20, 2025
U S Treasury Yields Dip As Federal Reserve Hints At Single 2025 Rate Decrease
May 20, 2025 -
 Strip The Duck Fan Outrage Over Jon Jones Latest Aspinall Remarks
May 20, 2025
Strip The Duck Fan Outrage Over Jon Jones Latest Aspinall Remarks
May 20, 2025
