Novavax COVID-19 Vaccine Gets FDA Nod, But With Unusual Usage Constraints
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Gets FDA Nod, but with Unusual Usage Constraints
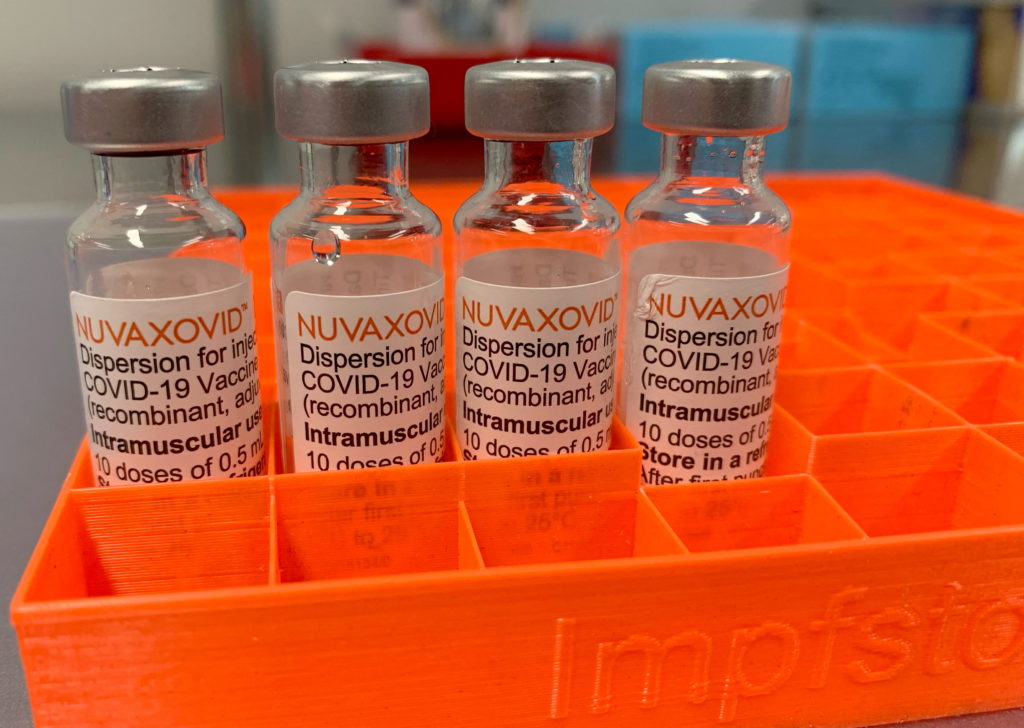
The FDA has finally granted approval to the Novavax COVID-19 vaccine, Nuvaxovid, but its entry into the US market is marked by unusual usage restrictions. This development, while welcomed by some, raises questions about the vaccine's role in the current landscape of COVID-19 vaccination. The approval comes after considerable delay and amidst a shifting public health focus.
Limited Authorization: A Strategic Shift?
Unlike other authorized COVID-19 vaccines, Nuvaxovid's approval isn't a blanket authorization for widespread use. The FDA's decision to grant Emergency Use Authorization (EUA) instead of full licensure, coupled with specific usage constraints, suggests a more targeted approach. This strategy seems to prioritize offering an alternative for individuals hesitant to receive mRNA vaccines, rather than positioning Novavax as a primary contender.
Why the Conditional Approval?
The FDA's decision is likely influenced by several factors. The relatively late arrival of Novavax on the scene means it faces a less urgent need compared to when other vaccines were initially authorized. Moreover, the efficacy data, while positive, might not be as compelling compared to the established mRNA vaccines, particularly regarding newer variants. The specific usage constraints may be a reflection of ongoing data collection and analysis, intended to refine the understanding of the vaccine's long-term effectiveness and safety profile.
Who is Nuvaxovid for?
The FDA's limited authorization points towards a specific target population: individuals who cannot or will not receive mRNA vaccines (Pfizer-BioNTech and Moderna) due to pre-existing conditions or personal preferences. This highlights a key aspect of the public health strategy – providing options to maximize vaccination coverage. This approach acknowledges vaccine hesitancy and aims to bridge the gap for those who might have been otherwise excluded from vaccination programs.
Understanding the Usage Constraints:
While the specific constraints may evolve with further data analysis, the current limitations likely revolve around:
- Age Restrictions: The EUA might initially specify age groups where the benefit-risk profile is most favorable based on available clinical trial data.
- Prior Infection History: The vaccine's effectiveness might be evaluated differently for individuals with prior COVID-19 infection.
- Specific Risk Groups: The approval might prioritize certain vulnerable populations with specific health concerns where the advantages outweigh potential risks.
The Future of Novavax in the US Market:
The FDA's conditional approval of the Novavax vaccine presents a complex scenario. While providing an alternative for a segment of the population, its limited authorization suggests it won't significantly alter the dominant role of mRNA vaccines. The future success of Nuvaxovid will depend on public acceptance, further clinical data supporting its efficacy against emerging variants, and a clear communication strategy from both the FDA and Novavax. Further research will be crucial to establish its long-term efficacy and safety profile and to determine its place in the ongoing fight against COVID-19.
Call to Action:
Consult your healthcare provider to discuss the suitability of the Novavax vaccine for your individual circumstances. Staying informed about the latest COVID-19 vaccine updates is crucial for making informed healthcare decisions. For up-to-date information, refer to the and .

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, But With Unusual Usage Constraints. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Trumps Immigration Policy Upheld Supreme Court Allows Termination Of Venezuelan Migrant Protections
May 20, 2025
Trumps Immigration Policy Upheld Supreme Court Allows Termination Of Venezuelan Migrant Protections
May 20, 2025 -
 Watch Now The Intense Ww 1 Drama Starring Daniel Craig Cillian Murphy And Tom Hardy
May 20, 2025
Watch Now The Intense Ww 1 Drama Starring Daniel Craig Cillian Murphy And Tom Hardy
May 20, 2025 -
 Fdas Approval Of Novavax Covid 19 Vaccine Includes Unusual Restrictions
May 20, 2025
Fdas Approval Of Novavax Covid 19 Vaccine Includes Unusual Restrictions
May 20, 2025 -
 Philadelphia Eagles Lock Up Head Coach Nick Sirianni With New Deal
May 20, 2025
Philadelphia Eagles Lock Up Head Coach Nick Sirianni With New Deal
May 20, 2025 -
 Intense Solar Flare Triggers Radio Outages Across Europe Asia And The Middle East Watch Video
May 20, 2025
Intense Solar Flare Triggers Radio Outages Across Europe Asia And The Middle East Watch Video
May 20, 2025
