FDA's Approval Of Novavax COVID-19 Vaccine Includes Unusual Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
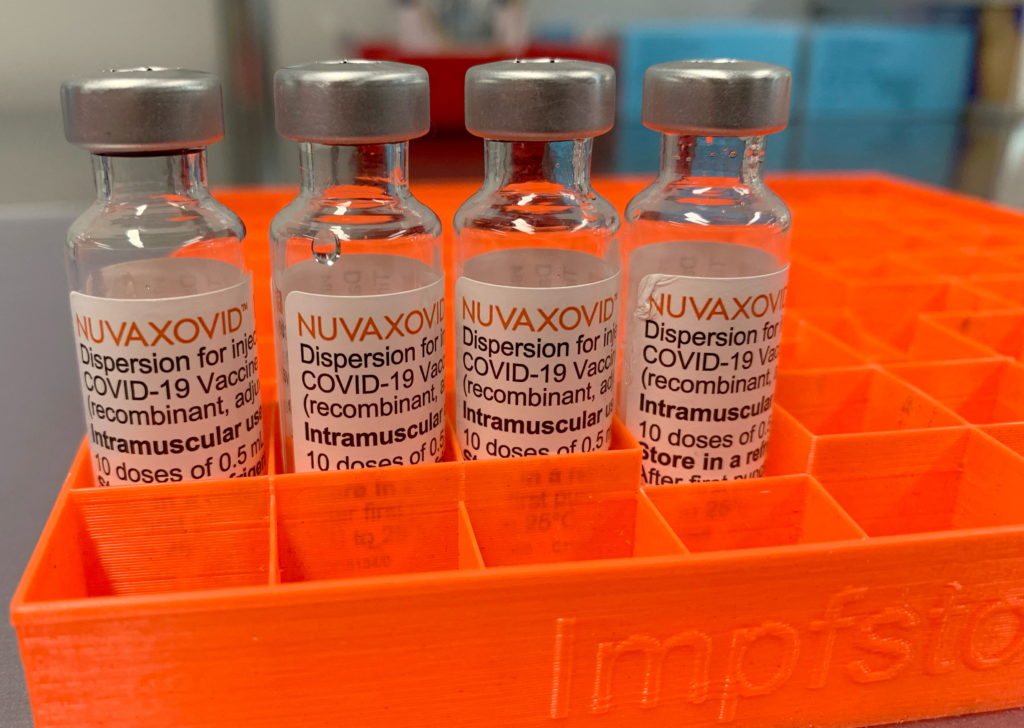
FDA Approves Novavax COVID-19 Vaccine, But with Unusual Restrictions
The FDA's approval of the Novavax COVID-19 vaccine, Nuvaxovid, has been met with a mix of relief and confusion. While the approval marks a significant step forward in the fight against the pandemic, the inclusion of unusual restrictions has raised eyebrows and sparked debate among health experts and the public. This landmark decision, announced [Insert Date of Announcement], comes with caveats that significantly limit its immediate impact and raise questions about its future role in the vaccination landscape.
What Makes the Novavax Approval Unusual?
Unlike previously authorized COVID-19 vaccines, Nuvaxovid's approval comes with several limitations. The most significant restriction is the limited age range. The FDA has only approved the vaccine for individuals aged 18 years and older. This contrasts sharply with other vaccines, like Pfizer-BioNTech and Moderna, which are authorized for younger age groups. This restricted age range significantly impacts the vaccine's potential reach and utility.
Furthermore, the FDA's approval is accompanied by a unique authorization process. Unlike the Emergency Use Authorization (EUA) granted to other vaccines, Nuvaxovid received a full Biologics License Application (BLA) approval. However, this approval is not without conditions. The FDA has imposed stringent manufacturing oversight requirements, raising concerns about potential production delays and logistical challenges. This level of scrutiny could impact the vaccine's availability and distribution.
Why the Restrictions?
The FDA has cited several reasons for the unusual restrictions. While the vaccine's efficacy and safety were deemed acceptable, the agency highlights the need for continued monitoring of its long-term effects. The unusual manufacturing requirements stem from the need to ensure consistent quality and safety standards. The FDA emphasizes a commitment to rigorous oversight, prioritizing public health and safety above all else.
The Implications for Public Health
The limited availability and restricted age range pose challenges for public health efforts. With many individuals already vaccinated with other COVID-19 vaccines, the demand for Nuvaxovid might be limited, particularly given the existing options. However, the vaccine could still offer a valuable alternative for individuals who have concerns about mRNA vaccines, providing a protein-subunit based option.
The FDA's decision underscores the complex regulatory landscape surrounding vaccine approvals. While full approval offers greater confidence in the vaccine's safety and efficacy, the stringent conditions attached to Nuvaxovid's approval highlight the ongoing need for vigilance and meticulous oversight in the development and deployment of vaccines.
Looking Ahead:
The future of Nuvaxovid remains uncertain. Its success will largely depend on overcoming the challenges posed by the FDA's restrictions. Increased production capacity, expansion of the approved age range, and effective public health communication will be crucial to maximizing the vaccine's potential impact. Further research and monitoring of long-term effects will also be essential in building confidence and widening its adoption. The coming months will be critical in determining whether Nuvaxovid can establish a significant role in the ongoing global vaccination efforts.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, vaccine restrictions, protein-subunit vaccine, Biologics License Application (BLA), Emergency Use Authorization (EUA), vaccine safety, vaccine efficacy, public health, mRNA vaccines.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA's Approval Of Novavax COVID-19 Vaccine Includes Unusual Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Impact Of Feds Rate Cut Projection On Us Treasury Yields
May 20, 2025
Impact Of Feds Rate Cut Projection On Us Treasury Yields
May 20, 2025 -
 New Peaky Blinders Series Confirmed What To Expect From The Next Chapter
May 20, 2025
New Peaky Blinders Series Confirmed What To Expect From The Next Chapter
May 20, 2025 -
 Jenn Sterger Recounts Fallout From Brett Favre Sexting Scandal
May 20, 2025
Jenn Sterger Recounts Fallout From Brett Favre Sexting Scandal
May 20, 2025 -
 Enhancing Tourist Behavior In Bali A Call For International Assistance
May 20, 2025
Enhancing Tourist Behavior In Bali A Call For International Assistance
May 20, 2025 -
 Putin Demonstrates Trumps Unimportance On The World Stage
May 20, 2025
Putin Demonstrates Trumps Unimportance On The World Stage
May 20, 2025
