Novavax COVID-19 Vaccine Gets FDA Nod, But With Uncommon Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
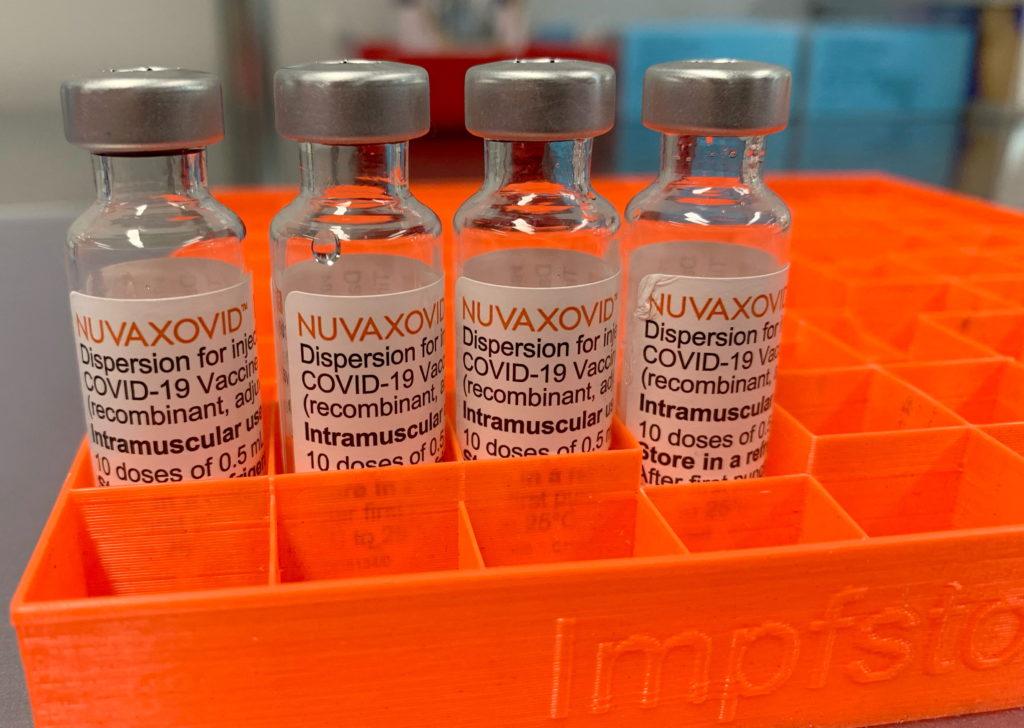
Novavax COVID-19 Vaccine Gets FDA Nod, but with Uncommon Usage Restrictions
The FDA has finally granted full approval to the Novavax COVID-19 vaccine, Nuvaxovid, marking a significant milestone in the fight against the pandemic. However, the approval comes with some unusual caveats that limit its widespread use, raising questions about its practical impact.
The approval, announced on [Insert Date of Announcement], grants Nuvaxovid full licensure for individuals 18 years and older. This is a crucial step, as previously, the vaccine was only available under an Emergency Use Authorization (EUA). This full approval signifies a rigorous review process by the FDA, bolstering confidence in its safety and efficacy. However, the FDA's decision also includes some unexpected limitations.
Restricted Usage: Why the Cautious Approach?
Unlike other widely used COVID-19 vaccines, Nuvaxovid's full approval isn't a blanket green light. The FDA's decision to grant full approval alongside these restrictions is likely due to several factors:
- Lower Uptake Compared to mRNA Vaccines: The mRNA vaccines from Pfizer-BioNTech and Moderna gained significant market share early in the vaccination rollout. Novavax’s later entry into the market meant it faced a tougher battle for adoption.
- Specific Efficacy Data: While the vaccine demonstrated efficacy against COVID-19, the data might not be as robust as that for the mRNA vaccines, especially against newer variants. The FDA may have placed restrictions to ensure appropriate targeting of the vaccine's usage.
- Post-Market Surveillance: The FDA often uses post-market surveillance to further monitor the safety and effectiveness of newly approved drugs and vaccines. This phased approach allows for continuous data collection and adjustments as needed.
The exact nature of these restrictions hasn't been fully detailed, but reports suggest that the FDA's approval might prioritize certain populations or contexts for Nuvaxovid use. This could include individuals with specific allergies or those who might prefer a protein-based vaccine over mRNA alternatives. More information regarding the specific criteria is expected to be released in the coming days.
What Does This Mean for the Future of COVID-19 Vaccination?
The FDA's decision highlights the complexities of vaccine rollout and the ongoing need for adaptable strategies. While full approval is a positive step for Novavax, the associated restrictions underscore the nuanced considerations involved in public health decision-making.
This development also raises several crucial questions:
- Will insurance companies cover the vaccine without restrictions? Insurance coverage is a vital factor influencing vaccine accessibility.
- How will healthcare providers navigate these usage guidelines? Clear communication and training for healthcare professionals are paramount.
- What impact will these restrictions have on vaccination rates? Public perception and understanding of the restrictions are key to ensuring continued vaccination efforts.
The availability of diverse vaccine options remains crucial for a comprehensive public health strategy. Nuvaxovid, with its protein-based technology, offers an alternative for those who might be hesitant about mRNA vaccines. However, the FDA's cautious approach highlights the ongoing need for careful monitoring and data-driven decision-making in the ever-evolving landscape of the COVID-19 pandemic. We will continue to update this article as more information becomes available.
Stay informed on the latest COVID-19 vaccine news by subscribing to our newsletter [link to newsletter signup].

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, But With Uncommon Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Bitcoin Etf Investment Boom 5 Billion And Counting Whats Next
May 20, 2025
Bitcoin Etf Investment Boom 5 Billion And Counting Whats Next
May 20, 2025 -
 International Support Urged For Balis Tourist Safety And Conduct
May 20, 2025
International Support Urged For Balis Tourist Safety And Conduct
May 20, 2025 -
 Tom Aspinall Talks Stall Jon Jones Hints At Retirement Ufc Fans React
May 20, 2025
Tom Aspinall Talks Stall Jon Jones Hints At Retirement Ufc Fans React
May 20, 2025 -
 Post Shanghai Upgrade Investors Pour 200 Million Into Ethereum Funds
May 20, 2025
Post Shanghai Upgrade Investors Pour 200 Million Into Ethereum Funds
May 20, 2025 -
 Nick Siriannis Multi Year Extension A Win For The Philadelphia Eagles
May 20, 2025
Nick Siriannis Multi Year Extension A Win For The Philadelphia Eagles
May 20, 2025
