Novavax COVID-19 Vaccine Gets FDA Nod, But With Significant Usage Constraints
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Gets FDA Nod, But with Significant Usage Constraints
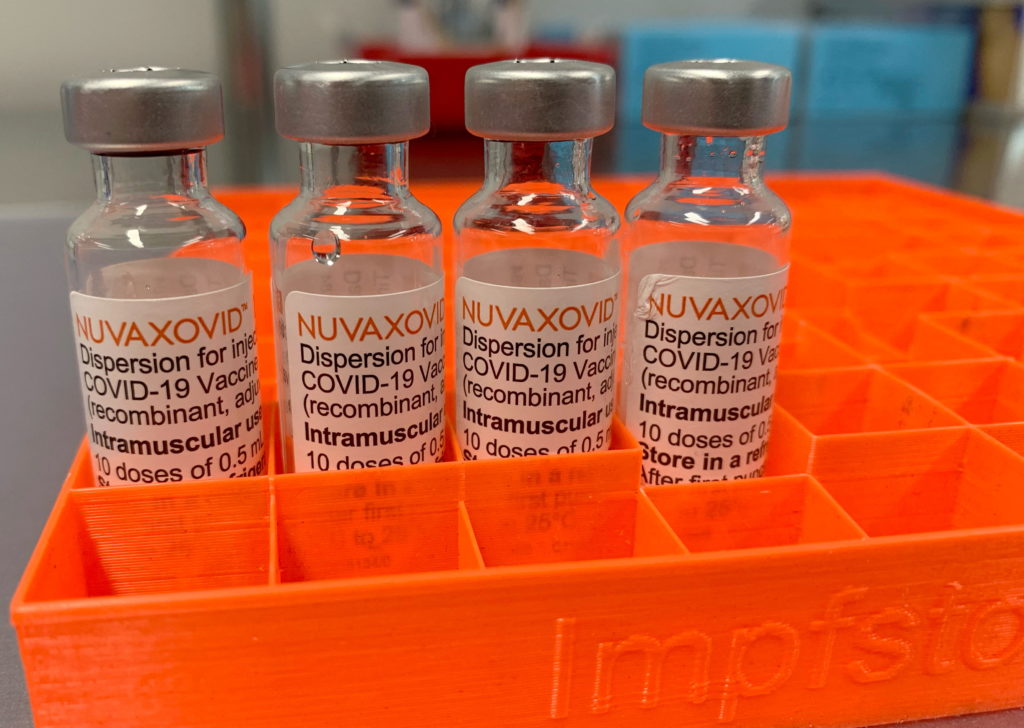
The FDA has finally approved the Novavax COVID-19 vaccine, Nuvaxovid, but its rollout faces hurdles due to limited demand and logistical challenges. This approval, while a victory for Novavax, comes with significant caveats that may limit its widespread use in the United States.
The long-awaited approval of Nuvaxovid, a protein subunit vaccine, offers an alternative to mRNA vaccines like Pfizer-BioNTech and Moderna. Many were hoping it would provide a boost to vaccination rates, particularly among those hesitant to receive mRNA vaccines. However, the reality is far more nuanced.
Why the Limited Rollout?
Several factors contribute to the constrained usage of the Novavax vaccine:
-
Waning Demand: The initial surge in COVID-19 vaccinations has subsided considerably. With widespread availability of other vaccines and a decrease in severe cases, the demand for a new vaccine is relatively low. Many individuals already have immunity through prior infection or vaccination.
-
Logistical Challenges: The FDA approval comes at a time when the US is already well-stocked with mRNA vaccines. Distributing and storing Nuvaxovid requires a different logistical infrastructure, potentially adding complexities to an already established system.
-
Efficacy Concerns: While effective, Nuvaxovid's efficacy against newer variants may be lower compared to updated mRNA boosters. This could further limit its appeal to those already vaccinated or boosted. [Link to CDC data on vaccine efficacy]
-
Competition from Established Vaccines: The established dominance of mRNA vaccines in the market makes it difficult for Novavax to gain significant market share. Healthcare providers may be less inclined to switch to a new vaccine with a potentially smaller pool of patients.
What Does This Mean for the Future of COVID-19 Vaccination?
The approval of the Novavax vaccine is a significant development, representing a technological diversity in the fight against COVID-19. However, its immediate impact on vaccination rates remains uncertain. The vaccine's role might be more significant in specific populations or regions with logistical needs that better align with its characteristics. It's possible Nuvaxovid will find more traction internationally where access to mRNA vaccines is limited.
The FDA's approval process, while rigorous, also highlights the complexities of vaccine rollout in a constantly evolving pandemic landscape. The agency's decision underscores the importance of continually evaluating vaccine efficacy and safety, and adapting strategies as new variants emerge and public health needs shift.
Looking Ahead: Will Novavax Find its Niche?
The success of Nuvaxovid will depend heavily on several factors: targeted marketing campaigns focusing on specific demographics, a streamlined distribution strategy, and potentially, the emergence of new variants against which it demonstrates superior efficacy. While the initial rollout might be constrained, the future role of Novavax's vaccine in the global fight against COVID-19 remains to be seen. This approval certainly broadens the options available, but only time will tell how significant its impact will be.
Call to Action: Stay informed about COVID-19 vaccines and consult your healthcare provider to discuss which vaccine is best for you. [Link to CDC website on COVID-19 vaccines]

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, But With Significant Usage Constraints. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 My 600 Lb Lifes Latonya Pottain Passes Away At 40 A Tragic Loss
May 20, 2025
My 600 Lb Lifes Latonya Pottain Passes Away At 40 A Tragic Loss
May 20, 2025 -
 Widespread Tornado Risk Severe Weather System Sweeping Across The Us
May 20, 2025
Widespread Tornado Risk Severe Weather System Sweeping Across The Us
May 20, 2025 -
 Investing In A Green Future The Role Of Clean Energy Tax Policy In America
May 20, 2025
Investing In A Green Future The Role Of Clean Energy Tax Policy In America
May 20, 2025 -
 Trump Calls For Immediate Russia Ukraine Peace Negotiations
May 20, 2025
Trump Calls For Immediate Russia Ukraine Peace Negotiations
May 20, 2025 -
 Investing In The Future The Role Of Medical And Scientific Research In Americas Prosperity
May 20, 2025
Investing In The Future The Role Of Medical And Scientific Research In Americas Prosperity
May 20, 2025
