Novavax COVID-19 Vaccine: FDA Grants Approval, But With Key Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
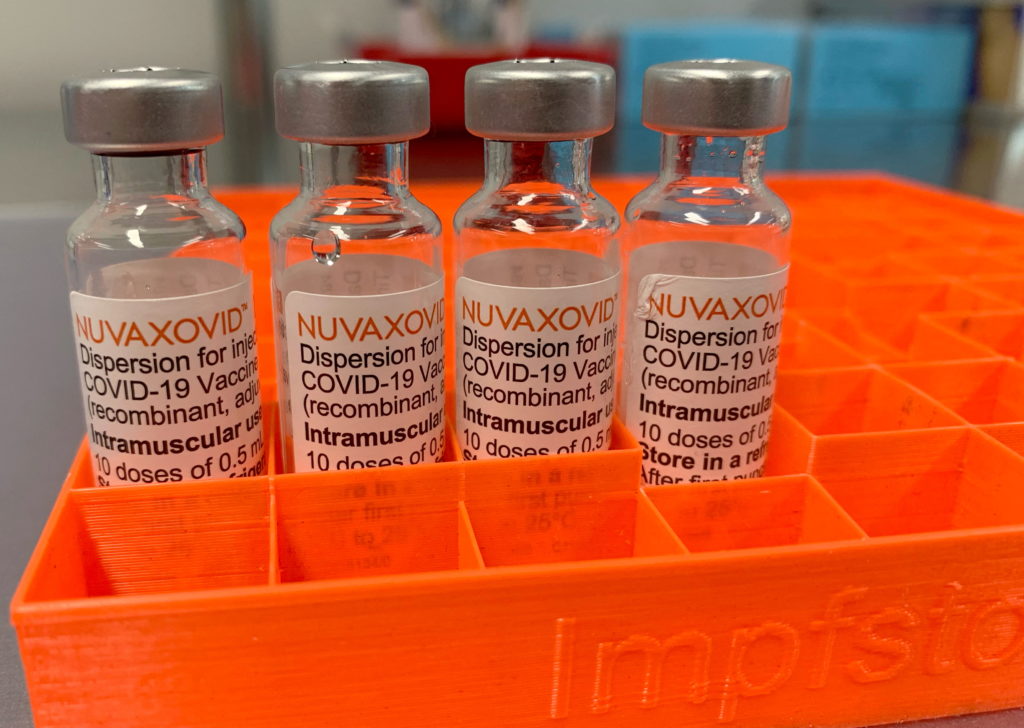
Novavax COVID-19 Vaccine: FDA Grants Approval, but with Key Restrictions
The FDA has granted Emergency Use Authorization (EUA) to the Novavax COVID-19 vaccine, Nuvaxovid, marking a significant development in the fight against the pandemic. However, this approval comes with key restrictions, raising questions about its widespread adoption. This news has sent ripples through the medical community and sparked considerable debate amongst public health officials and the general public.
What Makes Novavax Different?
Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, or the viral vector vaccine from Johnson & Johnson, Novavax uses a more traditional protein subunit technology. This technology has been used for decades in other vaccines, potentially appealing to individuals hesitant about the newer mRNA approaches. The vaccine works by introducing a lab-made version of the SARS-CoV-2 spike protein into the body, triggering an immune response. This familiar technology might alleviate some vaccine hesitancy, although further research is needed to assess its long-term efficacy and safety compared to existing vaccines.
FDA Approval: A Qualified Victory
While the FDA's approval is a welcome development, it's crucial to understand the accompanying limitations. The authorization is specifically for individuals aged 18 and older, excluding younger age groups. This is a key restriction compared to the broader age ranges covered by other authorized COVID-19 vaccines.
Furthermore, the FDA’s decision is contingent on ongoing monitoring of the vaccine's safety and efficacy profile. This means that continuous surveillance and data analysis will be crucial in determining its long-term role in the vaccination strategy.
Key Restrictions and Concerns:
- Limited Age Range: The vaccine's authorization is currently limited to adults aged 18 years and older. Further trials are needed to assess its safety and efficacy in younger populations.
- Manufacturing and Distribution: The initial rollout of the Novavax vaccine is expected to be limited, potentially hindering its immediate impact on vaccination rates globally. This raises concerns about accessibility and equitable distribution.
- Efficacy Compared to Other Vaccines: While clinical trials demonstrated a high level of efficacy against severe COVID-19, its performance compared to already-established vaccines needs continued evaluation, especially against emerging variants.
Looking Ahead: The Role of Novavax in the Pandemic Response
The arrival of the Novavax vaccine presents both opportunities and challenges. Its traditional technology might sway vaccine-hesitant individuals, potentially increasing overall vaccination rates. However, the restrictions surrounding its use, coupled with potential logistical hurdles, temper initial optimism. The long-term impact of the Novavax vaccine will depend on its accessibility, efficacy against emerging variants, and ongoing monitoring of its safety profile.
Further research and data collection will be critical in determining its ultimate role in the ongoing pandemic response and the future landscape of COVID-19 vaccination. The FDA's decision underscores the importance of continuing to evaluate and refine our strategies against this evolving virus. For more information on COVID-19 vaccines and vaccination recommendations, consult the .
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, EUA, protein subunit vaccine, vaccine hesitancy, vaccine efficacy, vaccine safety, pandemic response, COVID-19 vaccination, mRNA vaccine, viral vector vaccine, public health.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Grants Approval, But With Key Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Overcompensating A Coming Out Comedy Thats Both Funny And Thought Provoking
May 20, 2025
Overcompensating A Coming Out Comedy Thats Both Funny And Thought Provoking
May 20, 2025 -
 Marsh And Markrams Fifties Set Up 206 Run Target For Lsg
May 20, 2025
Marsh And Markrams Fifties Set Up 206 Run Target For Lsg
May 20, 2025 -
 Federal Reserves Rate Cut Prediction Effect On U S Treasury Yields
May 20, 2025
Federal Reserves Rate Cut Prediction Effect On U S Treasury Yields
May 20, 2025 -
 Nasa Warning Powerful Solar Flares Threaten Earths Power Grid
May 20, 2025
Nasa Warning Powerful Solar Flares Threaten Earths Power Grid
May 20, 2025 -
 Washington D C Hosts World Pride A Look At The Event And Its Political Context
May 20, 2025
Washington D C Hosts World Pride A Look At The Event And Its Political Context
May 20, 2025
