Novavax COVID-19 Vaccine: FDA Approval And Key Usage Restrictions Explained
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
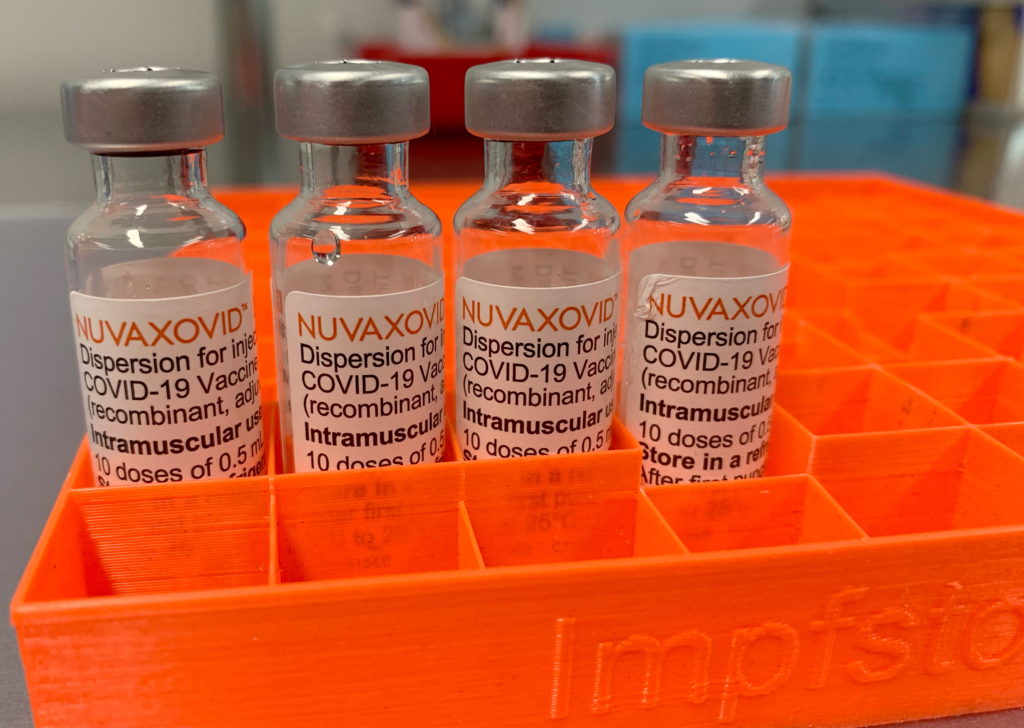
Novavax COVID-19 Vaccine: FDA Approval, Key Usage Restrictions, and What You Need to Know
The Novavax COVID-19 vaccine, Nuvaxovid (also known as Covovax internationally), has received FDA approval, offering another option in the fight against the virus. However, its use isn't completely unrestricted. Understanding the FDA's approval and the key limitations surrounding its usage is crucial for both healthcare providers and the public. This article clarifies the details, separating fact from speculation.
FDA Approval and Authorization:
The FDA granted Nuvaxovid Emergency Use Authorization (EUA) in July 2022, allowing its use in individuals 12 years of age and older. Crucially, the FDA's recent approval transitioned the vaccine from EUA to full licensure for individuals 18 years and older. This full approval signifies a rigorous review process, bolstering confidence in its safety and effectiveness. For those under 18, the vaccine remains under EUA. This distinction is important because the approval process for different age groups can vary slightly depending on available data.
How Does the Novavax Vaccine Work?
Unlike mRNA vaccines like Pfizer-BioNTech and Moderna, Novavax uses a more traditional protein subunit technology. This means the vaccine uses purified fragments of the virus's spike protein to trigger an immune response. This approach has been used in vaccines for other diseases for years, potentially appealing to individuals hesitant about mRNA technology. This difference in technology is a key factor in understanding the vaccine's profile and potential suitability for specific populations.
Key Usage Restrictions and Considerations:
While the FDA approval is a significant milestone, several factors influence the vaccine's recommended use:
- Age Restrictions: As mentioned, full licensure currently applies only to individuals 18 years and older. Those aged 12-17 can still receive the vaccine under the EUA.
- Allergies: Individuals with a history of severe allergic reactions to any component of the vaccine should avoid it. This includes specific components like polysorbate 80, often found in various medications and vaccines.
- Underlying Health Conditions: While generally safe, individuals with specific underlying health conditions should consult their physician before receiving the vaccination. This is standard procedure for any vaccine and allows for personalized risk assessment.
- Pregnancy and Breastfeeding: Data on the vaccine's safety during pregnancy and breastfeeding is still accumulating. Pregnant and breastfeeding individuals should discuss the risks and benefits with their healthcare provider before vaccination.
- Efficacy Against Variants: Like all COVID-19 vaccines, the effectiveness of Novavax against emerging variants may vary. Ongoing research continues to monitor its efficacy against circulating strains.
Comparing Novavax to Other COVID-19 Vaccines:
The Novavax vaccine offers an alternative to mRNA vaccines. This is valuable because it provides an option for individuals who may prefer a protein-subunit vaccine due to personal preference or concerns about mRNA technology. However, head-to-head comparisons with other vaccines in terms of efficacy and long-term protection are still ongoing and require further research. The offers more information on comparing various vaccine types.
Conclusion:
The FDA approval of the Novavax COVID-19 vaccine presents a valuable addition to the available options. Understanding the specific usage restrictions and considering individual health circumstances is crucial for making informed decisions. Consult your doctor or healthcare provider to determine if the Novavax vaccine is the right choice for you. Remember to stay informed about the latest updates and guidance from reliable sources like the CDC and FDA. Staying up-to-date on COVID-19 vaccine information remains essential for maintaining community health.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Approval And Key Usage Restrictions Explained. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 The Putin Trump Dynamic A Shift In Global Power
May 20, 2025
The Putin Trump Dynamic A Shift In Global Power
May 20, 2025 -
 Over 5 Billion Invested In Bitcoin Etfs Analyzing The Bold Market Moves
May 20, 2025
Over 5 Billion Invested In Bitcoin Etfs Analyzing The Bold Market Moves
May 20, 2025 -
 Trumps Policy Upheld Supreme Court Denies Protected Status To Venezuelan Migrants
May 20, 2025
Trumps Policy Upheld Supreme Court Denies Protected Status To Venezuelan Migrants
May 20, 2025 -
 Lsg Vs Srh Live Score Ipl 2025 Heated Confrontation Steals The Spotlight
May 20, 2025
Lsg Vs Srh Live Score Ipl 2025 Heated Confrontation Steals The Spotlight
May 20, 2025 -
 Tariff Showdown Trump Tells Walmart To Absorb Increased Costs
May 20, 2025
Tariff Showdown Trump Tells Walmart To Absorb Increased Costs
May 20, 2025
