Novavax COVID-19 Vaccine Approved By FDA With Limitations
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
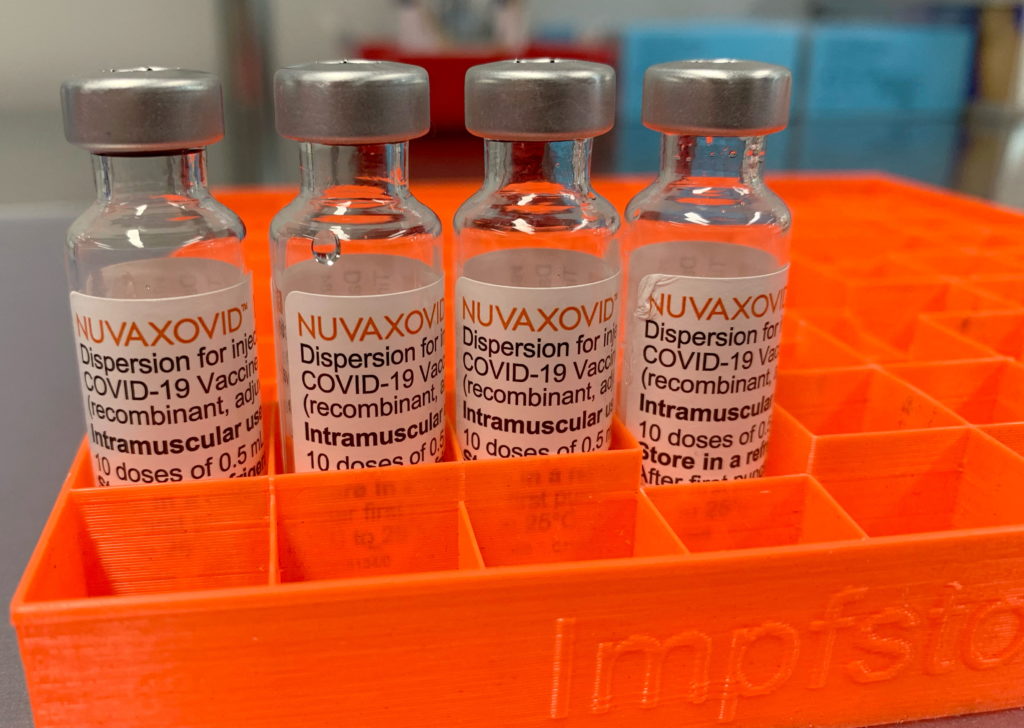
Novavax COVID-19 Vaccine Approved by FDA, but with Limitations
The FDA has finally granted Emergency Use Authorization (EUA) to the Novavax COVID-19 vaccine, Nuvaxovid, offering a new option for those seeking vaccination. However, this approval comes with significant limitations, prompting questions about its overall impact on the pandemic response. The agency's decision marks a significant development in the fight against COVID-19, but also highlights the evolving landscape of vaccine availability and choice.
A Different Approach: How Novavax Stands Apart
Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, or the viral vector vaccine from Johnson & Johnson, Novavax utilizes a more traditional protein subunit technology. This method involves using harmless pieces of the virus to trigger an immune response. This approach has long been familiar in vaccine development, potentially appealing to individuals hesitant about the newer mRNA technologies. Many believe this traditional method may alleviate some vaccine hesitancy.
Limitations and Conditions of the EUA
The FDA's approval isn't without caveats. The EUA is specifically for individuals 18 years of age and older. Furthermore, the agency highlights a lower efficacy rate compared to the mRNA vaccines, particularly against newer variants. While the vaccine demonstrated efficacy in clinical trials, its effectiveness against currently circulating variants remains a concern. The FDA emphasizes the importance of ongoing monitoring and data analysis to fully assess its long-term efficacy and safety.
Safety Profile and Side Effects:
The Novavax vaccine, like all vaccines, carries potential side effects. Commonly reported side effects include pain at the injection site, fatigue, headache, muscle pain, and joint pain. Serious side effects are rare but possible. The FDA's approval process involved a thorough review of safety data, but continued surveillance is crucial. For detailed information on potential side effects, consult the official FDA fact sheet and your healthcare provider.
The Role of Novavax in the Current COVID-19 Landscape
The addition of Novavax to the vaccine arsenal could prove beneficial. Its protein subunit technology might sway vaccine-hesitant individuals, increasing overall vaccination rates. However, given its limitations and the availability of other highly effective vaccines, its impact on the broader pandemic response remains to be seen. The vaccine’s efficacy against emerging variants is a key factor influencing its long-term role. The FDA’s continued monitoring will be essential in determining the vaccine's continued usefulness.
Looking Ahead: What Does this Mean for the Future?
The approval of the Novavax vaccine signifies a continued effort towards providing diverse vaccine options to combat COVID-19. While its limitations necessitate cautious optimism, its availability provides another tool in the fight against the virus. The ongoing need for booster shots and the potential for future variants will continue to shape the evolving role of Novavax and other vaccines in protecting public health.
Call to Action: Consult your healthcare provider to discuss whether the Novavax COVID-19 vaccine is the right choice for you, considering your individual health situation and risk factors. Stay informed about the latest updates on COVID-19 vaccines from reliable sources such as the CDC and FDA websites. [Link to CDC website] [Link to FDA website]

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Approved By FDA With Limitations. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Impact Of Feds 2025 Rate Cut Prediction On U S Treasury Yields
May 20, 2025
Impact Of Feds 2025 Rate Cut Prediction On U S Treasury Yields
May 20, 2025 -
 Fall Of Favre Director Discusses The Complexities Of Brett Favres Story
May 20, 2025
Fall Of Favre Director Discusses The Complexities Of Brett Favres Story
May 20, 2025 -
 Market Rally Continues S And P 500 Leads Gains As Investors Shake Off Moodys
May 20, 2025
Market Rally Continues S And P 500 Leads Gains As Investors Shake Off Moodys
May 20, 2025 -
 Messages Of Solidarity Pour In For President Biden After Cancer Announcement
May 20, 2025
Messages Of Solidarity Pour In For President Biden After Cancer Announcement
May 20, 2025 -
 Helldivers 2 Master Of Ceremony Warbond Drop Details For May 15th
May 20, 2025
Helldivers 2 Master Of Ceremony Warbond Drop Details For May 15th
May 20, 2025
