Novavax COVID-19 Vaccine Approved By FDA, But With Conditions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
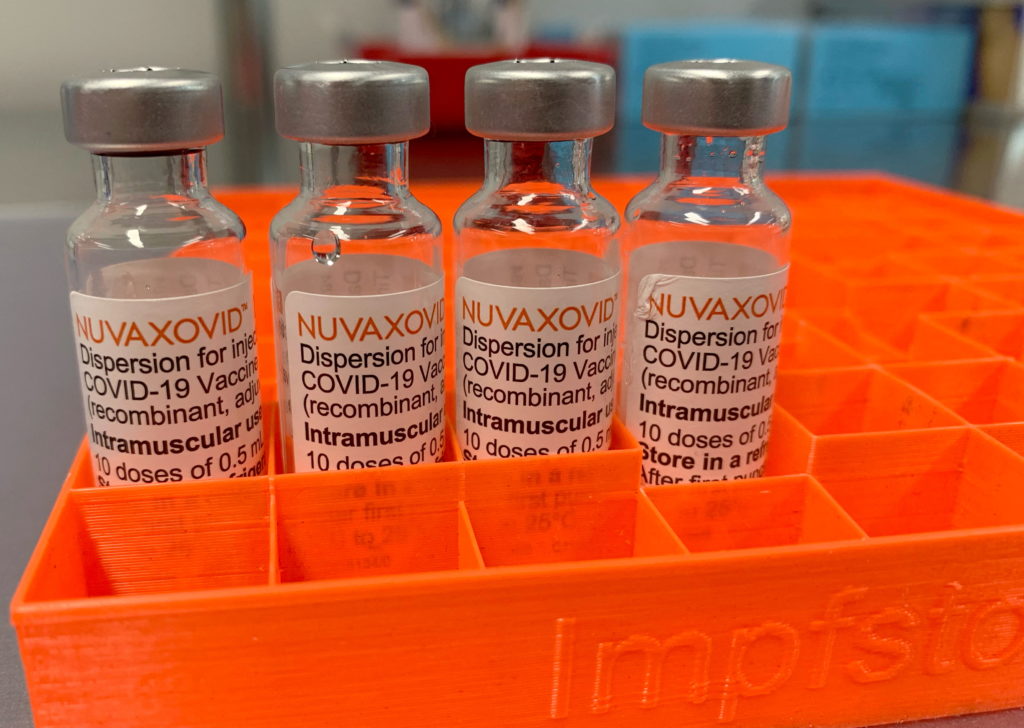
Novavax COVID-19 Vaccine Approved by FDA, But With Conditions
The FDA has finally granted approval to the Novavax COVID-19 vaccine, Nuvaxovid, but with several conditions attached. This long-awaited approval marks a significant development in the fight against the pandemic, offering an alternative vaccine option for those who may have hesitated to receive mRNA vaccines like Pfizer-BioNTech and Moderna. However, the conditional approval highlights ongoing concerns regarding the vaccine's efficacy and safety profile compared to its mRNA counterparts.
This article will delve into the specifics of the FDA's approval, the conditions imposed, the vaccine's potential impact, and what this means for the future of COVID-19 vaccination strategies.
Conditional Approval: What Does it Mean?
The FDA's approval is not a full, unconditional license. Instead, it's a conditional approval, meaning the agency will continue to monitor the vaccine's safety and efficacy closely. This approach allows for quicker access to the vaccine while ensuring ongoing scrutiny. The conditions imposed may include:
- Continued monitoring of adverse events: The FDA will rigorously track any reported side effects to assess the vaccine's long-term safety profile.
- Submission of further data: Novavax is required to provide additional data on the vaccine's efficacy and safety over time. This includes ongoing surveillance for rare adverse events.
- Manufacturing oversight: The FDA will maintain oversight of the manufacturing process to ensure consistent quality and safety.
This conditional approval reflects the FDA's commitment to both public health and rigorous scientific evaluation. It's a common practice for novel vaccines and therapies, allowing for a balanced approach between timely access and comprehensive safety monitoring.
Why the Delay and Conditional Approval?
The Novavax vaccine, a protein-subunit vaccine, utilizes a different technology than the widely used mRNA vaccines. This difference might account for some of the delays in its approval process. While the technology has been used successfully in other vaccines, the FDA needed to thoroughly assess its efficacy and safety profile specifically against COVID-19. The relatively lower efficacy rates compared to mRNA vaccines in clinical trials also likely contributed to the conditional approval.
What This Means for Individuals
The approval of the Novavax vaccine provides an important option for individuals who have been hesitant to receive mRNA vaccines due to concerns about their novel technology or potential side effects. The protein-subunit technology has a longer history and may be perceived as more familiar or less risky by some. However, it's crucial to remember that all vaccines undergo rigorous testing and approval processes, and both mRNA and protein-subunit vaccines are considered safe and effective.
Individuals considering the Novavax vaccine should consult with their healthcare provider to discuss their medical history and assess whether it’s the right choice for them. The decision should be based on an informed discussion with a medical professional, not solely on perceived differences in vaccine technology.
The Future of COVID-19 Vaccination
The approval of the Novavax vaccine adds another tool to the arsenal in the fight against COVID-19. While it may not entirely replace mRNA vaccines, it offers a valuable alternative, potentially increasing vaccination rates and contributing to herd immunity. The ongoing monitoring and data collection associated with the conditional approval will be essential in further understanding the long-term efficacy and safety profile of the vaccine and informing future vaccination strategies. This development underscores the importance of continued research and innovation in vaccine technology to combat evolving viral threats.
Call to Action: Consult your healthcare provider to discuss the COVID-19 vaccine that's best for you. Stay informed about the latest updates on COVID-19 vaccines and public health recommendations. For more information on COVID-19 vaccines, visit the .

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Approved By FDA, But With Conditions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Nfl News Eagles Extend Head Coach Nick Sirianni
May 20, 2025
Nfl News Eagles Extend Head Coach Nick Sirianni
May 20, 2025 -
 Heated Reaction Mma Community Responds To Jon Jones Strip The Duck Comment On Aspinall
May 20, 2025
Heated Reaction Mma Community Responds To Jon Jones Strip The Duck Comment On Aspinall
May 20, 2025 -
 Exclusive Interview The Director Of Netflixs Fall Of Favre On The Films Challenges
May 20, 2025
Exclusive Interview The Director Of Netflixs Fall Of Favre On The Films Challenges
May 20, 2025 -
 Fall Of Favre Director Untangling The Complex Legacy Of Brett Favre
May 20, 2025
Fall Of Favre Director Untangling The Complex Legacy Of Brett Favre
May 20, 2025 -
 Bitcoin Etf Investments Exceed 5 Billion Whats Driving The Growth
May 20, 2025
Bitcoin Etf Investments Exceed 5 Billion Whats Driving The Growth
May 20, 2025
