Limited FDA Approval: Novavax COVID-19 Vaccine Faces Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Limited FDA Approval: Novavax COVID-19 Vaccine Faces Usage Restrictions
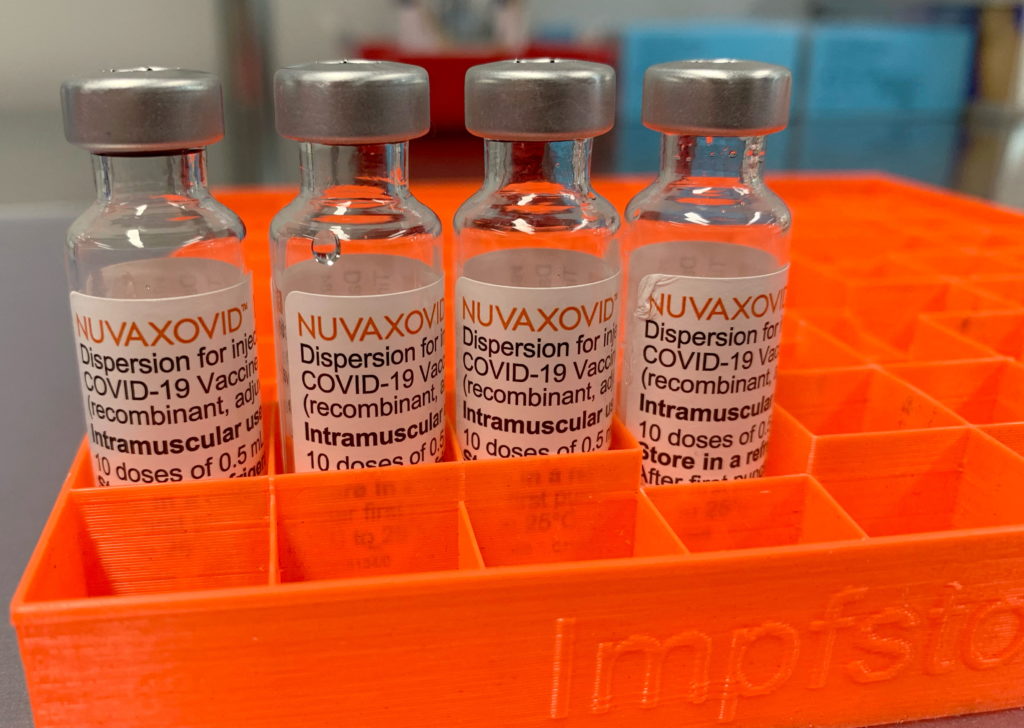
The Novavax COVID-19 vaccine, Nuvaxovid, has received limited approval from the FDA, raising questions about its future role in the ongoing pandemic. While a significant milestone for the company, the approval comes with substantial usage restrictions, limiting its potential impact compared to other widely available vaccines. This development underscores the complexities of vaccine rollout and the evolving landscape of the COVID-19 pandemic.
FDA Approval with Caveats:
The Food and Drug Administration (FDA) granted emergency use authorization (EUA) for Nuvaxovid, a protein subunit vaccine, for individuals 18 years of age and older. However, this approval is significantly narrower than that granted to mRNA vaccines like Pfizer-BioNTech and Moderna. The FDA's decision reflects concerns surrounding the vaccine's efficacy and safety profile compared to its competitors, highlighting the rigorous standards required for widespread adoption.
Why the Limited Approval?
Several factors contribute to the limited approval. While clinical trials demonstrated the vaccine's efficacy in preventing COVID-19, the data showed it was less effective than the mRNA vaccines, particularly against newer variants. Furthermore, production challenges and logistical hurdles have impacted the vaccine's timely rollout. The FDA's cautious approach prioritizes safety and efficacy, ensuring that only vaccines meeting stringent standards are authorized for use.
Usage Restrictions and Implications:
The limited approval translates to several key usage restrictions. Unlike mRNA vaccines which have been widely administered as boosters, Novavax's EUA currently only covers primary vaccination. This means it's not yet authorized as a booster shot, limiting its overall utility in a population already largely vaccinated or boosted with other vaccines. This restricted usage significantly impacts its market appeal and competitive position.
The Future of Novavax's COVID-19 Vaccine:
The future of Nuvaxovid remains uncertain. The company is currently pursuing full FDA licensure and further clinical trials to expand its authorized use to include booster shots and potentially younger age groups. The success of these endeavors will determine the vaccine's long-term impact on global vaccination efforts. The ongoing evolution of the virus and the emergence of new variants also present challenges, requiring ongoing monitoring and adaptation of vaccine strategies.
Alternatives and Current Vaccination Recommendations:
While Novavax offers another option, the CDC and other health organizations continue to recommend widely available mRNA vaccines (Pfizer-BioNTech and Moderna) as the primary choice for COVID-19 vaccination and boosting. These vaccines boast a superior safety and efficacy profile based on extensive real-world data and clinical trial results.
Conclusion:
The limited FDA approval of the Novavax COVID-19 vaccine signifies a cautious approach to ensuring vaccine safety and efficacy. While offering an alternative, its restricted usage and lower efficacy compared to other available vaccines limit its immediate impact. The ongoing development and future approvals will determine its long-term role in the fight against COVID-19. Consult your healthcare provider for personalized vaccination recommendations based on your individual health needs and risk factors.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Limited FDA Approval: Novavax COVID-19 Vaccine Faces Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 A J Perez On The Fallout From Untold Brett Favre Threats And The Future Of The Series
May 21, 2025
A J Perez On The Fallout From Untold Brett Favre Threats And The Future Of The Series
May 21, 2025 -
 Us Treasury Yield Decline Follows Feds 2025 Rate Cut Projection
May 21, 2025
Us Treasury Yield Decline Follows Feds 2025 Rate Cut Projection
May 21, 2025 -
 Overnight Storms And Heavy Rain Threaten North Carolina Severe Weather Warning Issued
May 21, 2025
Overnight Storms And Heavy Rain Threaten North Carolina Severe Weather Warning Issued
May 21, 2025 -
 Prostate Cancer And Gleason Score 9 What It Means For President Biden
May 21, 2025
Prostate Cancer And Gleason Score 9 What It Means For President Biden
May 21, 2025 -
 Trump To Intervene Planned Discussions With Putin And Zelensky To Secure Ukraine Cease Fire
May 21, 2025
Trump To Intervene Planned Discussions With Putin And Zelensky To Secure Ukraine Cease Fire
May 21, 2025
