Limited FDA Approval For Novavax COVID-19 Vaccine: What You Need To Know
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
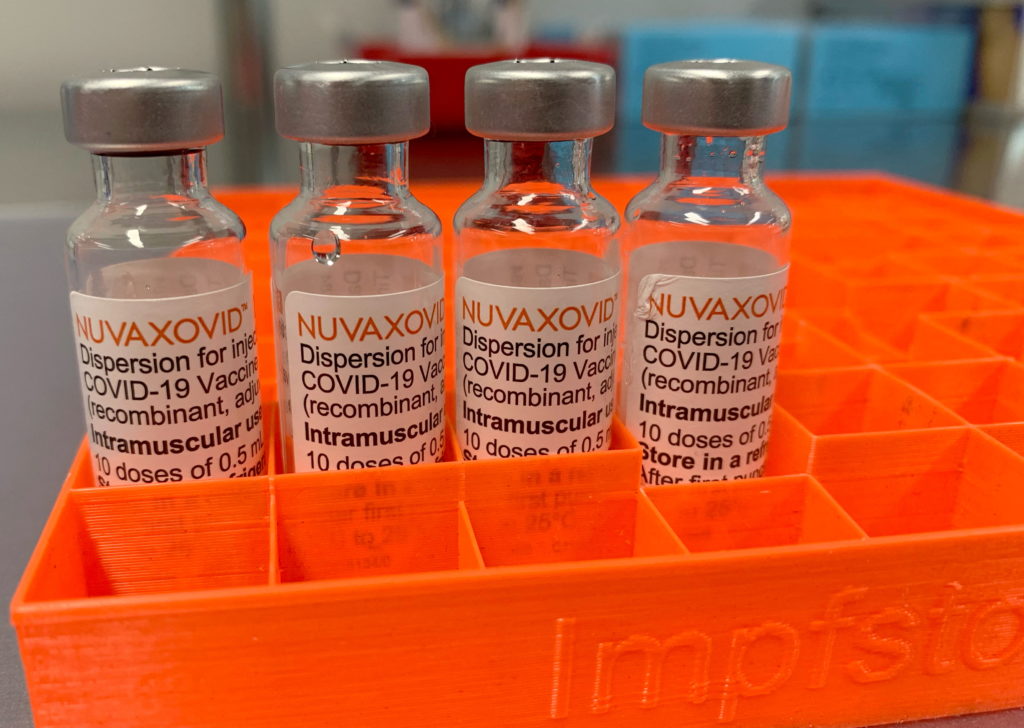
Limited FDA Approval for Novavax COVID-19 Vaccine: What You Need to Know
The US Food and Drug Administration (FDA) has granted limited approval for the Novavax COVID-19 vaccine, offering a new option for individuals seeking vaccination. This news is significant, particularly for those hesitant about mRNA vaccines like Pfizer-BioNTech and Moderna. But what does this "limited approval" mean, and what should you know before considering this vaccine? Let's delve into the details.
What is the Novavax COVID-19 Vaccine?
Unlike the mRNA vaccines, Novavax's Nuvaxovid uses a different technology. It's a protein subunit vaccine, meaning it uses a harmless piece of the virus's protein (the spike protein) to trigger an immune response. This method has been used for decades in other vaccines, such as the Hepatitis B vaccine, potentially making it a more familiar and comfortable choice for some.
What Does "Limited Approval" Mean?
The FDA's approval is for individuals 18 years of age and older. This is a significant step, granting the vaccine licensure under the agency's stringent standards. However, it's important to understand that this is not a full, unrestricted approval like some other vaccines have received. The FDA's decision is based on a comprehensive review of clinical trial data demonstrating the vaccine's safety and efficacy. Further post-market surveillance will continue to monitor its long-term effects.
Efficacy and Safety:
Clinical trials showed Novavax to be effective in preventing COVID-19. While the exact efficacy numbers might vary slightly depending on the specific study, the vaccine consistently demonstrated a significant reduction in symptomatic infections. Like all vaccines, potential side effects exist, but most are mild and temporary, such as soreness at the injection site, fatigue, and headache. Serious side effects are rare. The FDA thoroughly assessed these data before granting approval.
Why Choose Novavax?
The availability of Novavax provides an alternative for individuals who:
- Are hesitant about mRNA vaccines: The different technology might appeal to those with concerns about mRNA vaccines.
- Prefer a more traditional vaccine approach: The protein subunit technology is a familiar approach used in many established vaccines.
- Have specific medical needs or preferences: In some cases, a physician might recommend Novavax based on an individual's medical history.
What are the Next Steps?
If you're considering the Novavax vaccine, it's crucial to:
- Consult your doctor: Discuss your medical history, potential risks, and any concerns with your healthcare provider.
- Research the vaccine: Understand the technology, efficacy, and potential side effects. Reliable sources include the FDA website and the CDC website.
- Locate a vaccination site: Check with your local health department or pharmacy to find out where Novavax is available.
Conclusion:
The limited FDA approval of the Novavax COVID-19 vaccine is a positive development, providing another tool in the fight against the pandemic. While it offers a different approach compared to mRNA vaccines, it's essential to approach the decision to receive any vaccine with informed consent and consultation with a healthcare professional. Remember to stay informed about the latest updates from credible sources like the CDC and the FDA. This new vaccine option might be the right choice for you, but understanding the nuances of this limited approval is key.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Limited FDA Approval For Novavax COVID-19 Vaccine: What You Need To Know. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Clean Energy Tax Policy Analyzing The Economic Implications For America
May 20, 2025
Clean Energy Tax Policy Analyzing The Economic Implications For America
May 20, 2025 -
 Bali Seeks International Cooperation To Improve Tourist Behavior
May 20, 2025
Bali Seeks International Cooperation To Improve Tourist Behavior
May 20, 2025 -
 Claim Your Helldivers 2 Masters Of Ceremony Rewards Warbonds Available May 15th
May 20, 2025
Claim Your Helldivers 2 Masters Of Ceremony Rewards Warbonds Available May 15th
May 20, 2025 -
 Tom Aspinall Responds Fan Fury Over Jon Jones Strip The Duck Comment
May 20, 2025
Tom Aspinall Responds Fan Fury Over Jon Jones Strip The Duck Comment
May 20, 2025 -
 Record Bitcoin Etf Investments Exceed 5 Billion Market Trends And Analysis
May 20, 2025
Record Bitcoin Etf Investments Exceed 5 Billion Market Trends And Analysis
May 20, 2025
