FDA Clears Novavax COVID-19 Vaccine, Imposing Uncommon Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Clears Novavax COVID-19 Vaccine, but with Uncommon Usage Restrictions
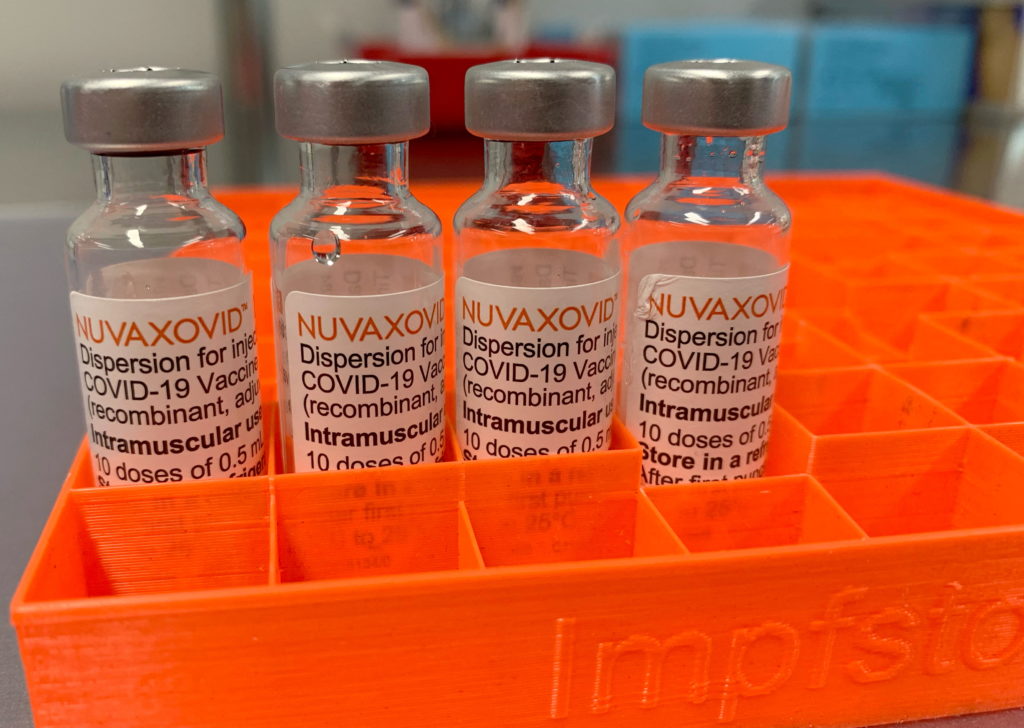
The FDA has granted Emergency Use Authorization (EUA) to Novavax's COVID-19 vaccine, Nuvaxovid, but with significant usage restrictions, marking a unique development in the ongoing pandemic response. This decision, announced [Insert Date of Announcement], comes after months of review and raises questions about the vaccine's place in the current landscape of readily available COVID-19 immunizations. While hailed by some as offering a different vaccine option, the imposed limitations highlight concerns regarding its efficacy and potential side effects.
What Makes Novavax Different?
Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, or the adenovirus-vector vaccines like Johnson & Johnson's Janssen, Nuvaxovid uses a more traditional protein subunit technology. This technology utilizes purified fragments of the virus's spike protein to trigger an immune response. This approach has historically been associated with fewer side effects in some individuals, leading to hopes that it could appeal to vaccine hesitant populations. However, the FDA's decision suggests that these benefits may be outweighed by other factors.
The Uncommon Restrictions: Why the Cautious Approach?
The FDA's EUA comes with several unusual limitations, primarily focusing on the vaccine's limited efficacy data compared to other authorized vaccines. Specifically, the FDA has:
- Restricted its use to adults 18 years and older: This excludes a significant portion of the population eligible for other COVID-19 vaccines.
- Emphasized its use only when other authorized or recommended COVID-19 vaccines are not accessible or acceptable: This points to Nuvaxovid's role as a secondary option rather than a primary choice.
- Included strong warnings about potential side effects: While the overall side effect profile is considered manageable, the FDA's emphasis on potential adverse reactions underscores a need for careful monitoring.
These restrictions contrast sharply with the broader authorizations granted to other COVID-19 vaccines. The FDA's cautious approach suggests a need for further data collection to fully understand Nuvaxovid's long-term efficacy and safety profile.
Implications for the COVID-19 Vaccination Campaign
The addition of Novavax's vaccine to the market, albeit with restrictions, offers a potentially valuable alternative for individuals who may be unable or unwilling to receive other vaccines. However, the limited usage guidelines raise questions about its overall impact on the ongoing vaccination efforts. The limited availability and restricted usage might not significantly alter the current vaccination landscape.
The FDA’s decision highlights the complexities of vaccine development and deployment, emphasizing the importance of rigorous scientific evaluation and the careful consideration of both efficacy and safety profiles. Further research and monitoring will be critical in determining the true role of Nuvaxovid in the global fight against COVID-19.
Looking Ahead: What's Next for Novavax?
Novavax will need to continue monitoring the vaccine's safety and efficacy post-authorization and provide additional data to the FDA to potentially expand its usage guidelines in the future. The success of Nuvaxovid will depend on its ability to address the concerns raised by the FDA and demonstrate its value within the existing vaccination landscape. Only time will tell if this novel approach to COVID-19 vaccination will gain significant traction.
Keywords: Novavax, COVID-19 vaccine, FDA, EUA, Nuvaxovid, protein subunit vaccine, vaccine restrictions, vaccine hesitancy, COVID-19 vaccination, vaccine safety, vaccine efficacy.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Clears Novavax COVID-19 Vaccine, Imposing Uncommon Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Thousands Of Spanish Holiday Rentals Face Closure New Ruling Impacts Bookings
May 20, 2025
Thousands Of Spanish Holiday Rentals Face Closure New Ruling Impacts Bookings
May 20, 2025 -
 Are Mortgage Refinance Rates Down May 19 2025 Analysis
May 20, 2025
Are Mortgage Refinance Rates Down May 19 2025 Analysis
May 20, 2025 -
 End Of Protected Status For Venezuelan Migrants Supreme Court Sides With Trump
May 20, 2025
End Of Protected Status For Venezuelan Migrants Supreme Court Sides With Trump
May 20, 2025 -
 Alexander Skarsgard Compares Brother Bills Nosferatu To True Blood
May 20, 2025
Alexander Skarsgard Compares Brother Bills Nosferatu To True Blood
May 20, 2025 -
 Confirmed A New Peaky Blinders Series Is Coming Featuring A Big Twist
May 20, 2025
Confirmed A New Peaky Blinders Series Is Coming Featuring A Big Twist
May 20, 2025
