FDA Approves Novavax COVID-19 Vaccine: Understanding The Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Approves Novavax COVID-19 Vaccine: Understanding the Usage Restrictions
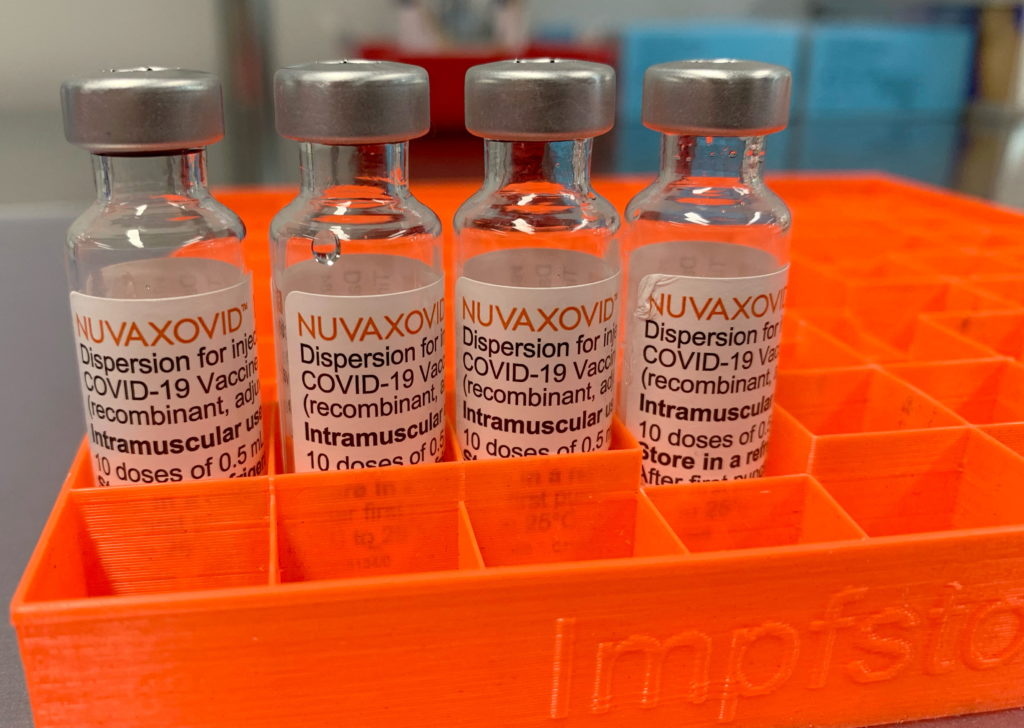
The FDA's approval of the Novavax COVID-19 vaccine, Nuvaxovid, marks a significant milestone in the fight against the pandemic. This protein-subunit vaccine offers a different approach compared to the mRNA vaccines from Pfizer-BioNTech and Moderna, providing an alternative for individuals who may have hesitated due to concerns about mRNA technology. However, understanding the vaccine's usage restrictions is crucial for both healthcare providers and the public.
A Different Approach: How Novavax Works
Unlike mRNA vaccines, which use messenger RNA to instruct cells to produce a viral protein, Novavax uses a more traditional method. Nuvaxovid contains a lab-made version of the spike protein found on the surface of the SARS-CoV-2 virus. This protein is not infectious and triggers an immune response in the body, teaching it to recognize and fight off the real virus. This approach may appeal to individuals who were hesitant about mRNA technology, offering a familiar vaccine platform.
FDA Approval and Authorized Use: Key Differences
It's important to clarify the distinction between "approval" and "emergency use authorization" (EUA). While Novavax initially received EUA, the recent FDA approval signifies a more rigorous review process and confirmation of its safety and effectiveness. This approval, however, comes with specific usage restrictions.
Understanding the Usage Restrictions
The FDA approval is for individuals 18 years of age and older. This is a key restriction, as some other COVID-19 vaccines have been authorized for younger age groups. Further research is needed to determine the vaccine's safety and efficacy in younger populations.
Other important considerations include:
- Allergic Reactions: As with any vaccine, individuals with a history of severe allergic reactions to any vaccine component should consult with their healthcare provider before receiving Nuvaxovid. This includes any known allergies to the components listed in the vaccine's package insert.
- Pre-existing Conditions: While generally safe, individuals with pre-existing medical conditions should discuss the vaccine with their doctor to assess any potential risks or interactions.
- Efficacy Data: While effective, the efficacy of Novavax may differ slightly from other authorized COVID-19 vaccines. The FDA approval is based on extensive clinical trial data, but individual responses can vary.
- Booster Shots: Information regarding booster shots and their necessity for Nuvaxovid is continually evolving. Stay updated with guidance from the CDC and your healthcare provider.
Finding Novavax: Access and Availability
The availability of the Novavax vaccine may vary depending on location. Check with your local health department, healthcare provider, or pharmacy to determine availability in your area. You can also utilize online resources like the to find vaccine locations near you.
Conclusion: Informed Choices are Key
The FDA approval of the Novavax COVID-19 vaccine provides a valuable alternative for individuals seeking protection against COVID-19. However, understanding the usage restrictions is crucial for making an informed decision. Always consult with your healthcare provider to determine if the Novavax vaccine is the right choice for you, considering your individual health history and circumstances. Staying updated on the latest information from reputable sources like the CDC and FDA is also essential in navigating this evolving landscape. Remember, vaccination remains a vital tool in the ongoing effort to control the pandemic.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approves Novavax COVID-19 Vaccine: Understanding The Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Messages Of Support Flood In For President Biden After Cancer Announcement
May 20, 2025
Messages Of Support Flood In For President Biden After Cancer Announcement
May 20, 2025 -
 Clean Energy Taxes Economic Impacts And The Path Forward For The Us
May 20, 2025
Clean Energy Taxes Economic Impacts And The Path Forward For The Us
May 20, 2025 -
 New Streaming Release A Wwi Epic With Daniel Craig Cillian Murphy And Tom Hardy
May 20, 2025
New Streaming Release A Wwi Epic With Daniel Craig Cillian Murphy And Tom Hardy
May 20, 2025 -
 Eagles Reward Sirianni With Contract Extension Following Super Bowl Victory
May 20, 2025
Eagles Reward Sirianni With Contract Extension Following Super Bowl Victory
May 20, 2025 -
 Jamie Lee Curtis Discusses Her Lasting Connection With Lindsay Lohan After The Hit Film
May 20, 2025
Jamie Lee Curtis Discusses Her Lasting Connection With Lindsay Lohan After The Hit Film
May 20, 2025
