FDA Approval For Novavax COVID-19 Vaccine: Key Restrictions Explained
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Approval for Novavax COVID-19 Vaccine: Key Restrictions Explained
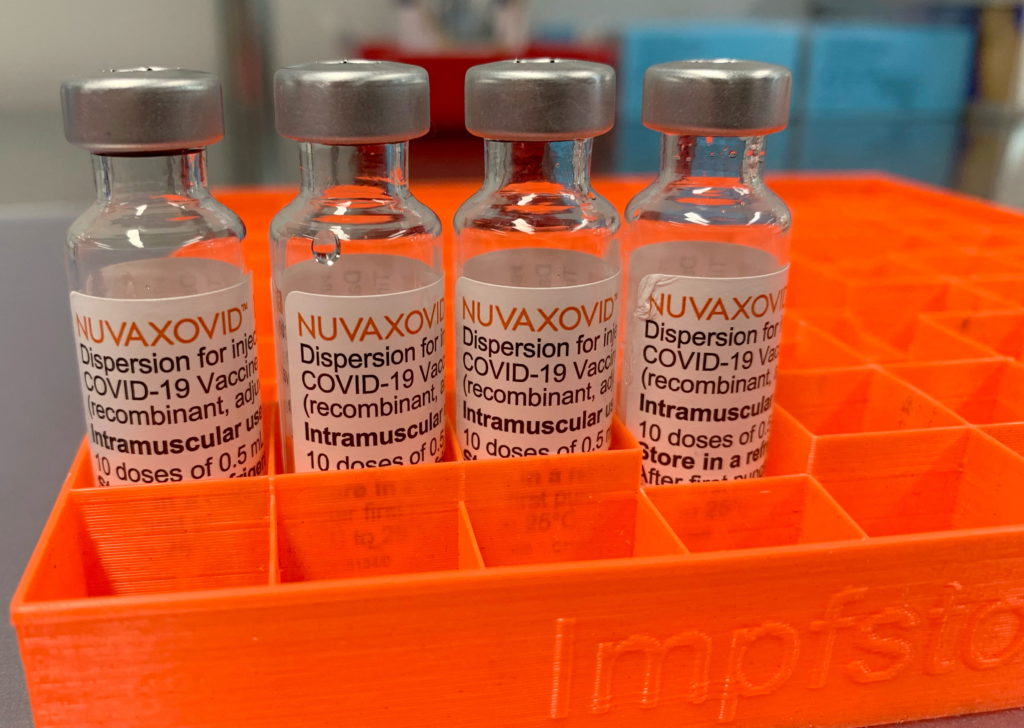
The FDA's approval of the Novavax COVID-19 vaccine, Nuvaxovid, marked a significant milestone in the fight against the pandemic. However, this approval isn't a blanket green light. Understanding the key restrictions surrounding its use is crucial for both healthcare professionals and the public. This article will break down the specifics, clarifying who can and cannot receive the vaccine and why.
What is Nuvaxovid?
Nuvaxovid, developed by Novavax, is a protein subunit vaccine. Unlike mRNA vaccines like Pfizer-BioNTech and Moderna, it uses a different technology. It introduces a harmless piece of the virus (the spike protein) to trigger an immune response. This protein-based approach has been a traditional method in vaccine development, potentially appealing to individuals hesitant about mRNA technology. [Link to CDC information on vaccine types]
FDA Approval: A Qualified Victory
While the FDA granted approval, it's important to note that this isn't identical to the full approvals granted to other COVID-19 vaccines. The approval is specifically for individuals 18 years of age and older. This age restriction is a key point to remember.
Key Restrictions and Considerations:
-
Age Limitation: Currently, Nuvaxovid is only approved for individuals 18 years and older. This means it's not authorized for use in children or adolescents. Further studies are needed to determine its safety and efficacy in younger populations.
-
Limited Data on Variants: While effective against the original COVID-19 strain, data on the vaccine's effectiveness against newer variants, such as Omicron subvariants, is still emerging and requires ongoing monitoring. [Link to a reputable source discussing vaccine efficacy against variants].
-
Potential Side Effects: Like all vaccines, Nuvaxovid carries potential side effects. Common side effects include pain at the injection site, fatigue, headache, muscle aches, and joint pain. Severe side effects are rare but possible. Individuals should consult their doctor if they experience any concerning symptoms after receiving the vaccine. [Link to information on reported side effects from a reliable source like the CDC or FDA].
Who Should Consider Nuvaxovid?
Individuals 18 and older who:
- Are hesitant about mRNA vaccines.
- Have had adverse reactions to other COVID-19 vaccines.
- Prefer a protein subunit vaccine.
Should consult with their healthcare provider to determine if Nuvaxovid is the right choice for them. It is crucial to discuss your medical history and any concerns before receiving any vaccination.
The Future of Nuvaxovid:
Novavax is actively pursuing authorization for use in younger age groups and conducting further research on its efficacy against emerging variants. The long-term effectiveness and safety of the vaccine will continue to be monitored.
Conclusion:
The FDA approval of Novavax's COVID-19 vaccine represents a valuable addition to the available vaccination options. However, understanding the current limitations, particularly the age restriction and ongoing research regarding variant efficacy, is crucial for informed decision-making. Always consult with your healthcare provider to determine the most appropriate vaccination strategy for your individual needs and circumstances. Staying informed about the latest developments in COVID-19 vaccination is vital for protecting yourself and your community.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, protein subunit vaccine, vaccine restrictions, vaccine side effects, vaccine efficacy, COVID-19 variants, mRNA vaccine, vaccination, healthcare
Call to Action (subtle): Talk to your doctor today about which COVID-19 vaccine is right for you.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Approval For Novavax COVID-19 Vaccine: Key Restrictions Explained. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Must Win Match For Lsg Srhs 206 Run Total Built On Marsh And Markrams Performances
May 20, 2025
Must Win Match For Lsg Srhs 206 Run Total Built On Marsh And Markrams Performances
May 20, 2025 -
 Peaky Blinders Future Creator Announces New Series Heralding A Different Approach
May 20, 2025
Peaky Blinders Future Creator Announces New Series Heralding A Different Approach
May 20, 2025 -
 Bitcoin Etf Investments Exceed 5 Billion Whats Driving The Growth
May 20, 2025
Bitcoin Etf Investments Exceed 5 Billion Whats Driving The Growth
May 20, 2025 -
 Tornado Watch Lifted In North Texas Dallas Weather Report
May 20, 2025
Tornado Watch Lifted In North Texas Dallas Weather Report
May 20, 2025 -
 Helldivers 2 Get Masters Of Ceremony Warbonds Starting May 15th
May 20, 2025
Helldivers 2 Get Masters Of Ceremony Warbonds Starting May 15th
May 20, 2025
