Conditional FDA Approval: The Novavax COVID-19 Vaccine And Its Limitations
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Conditional FDA Approval: The Novavax COVID-19 Vaccine and its Limitations
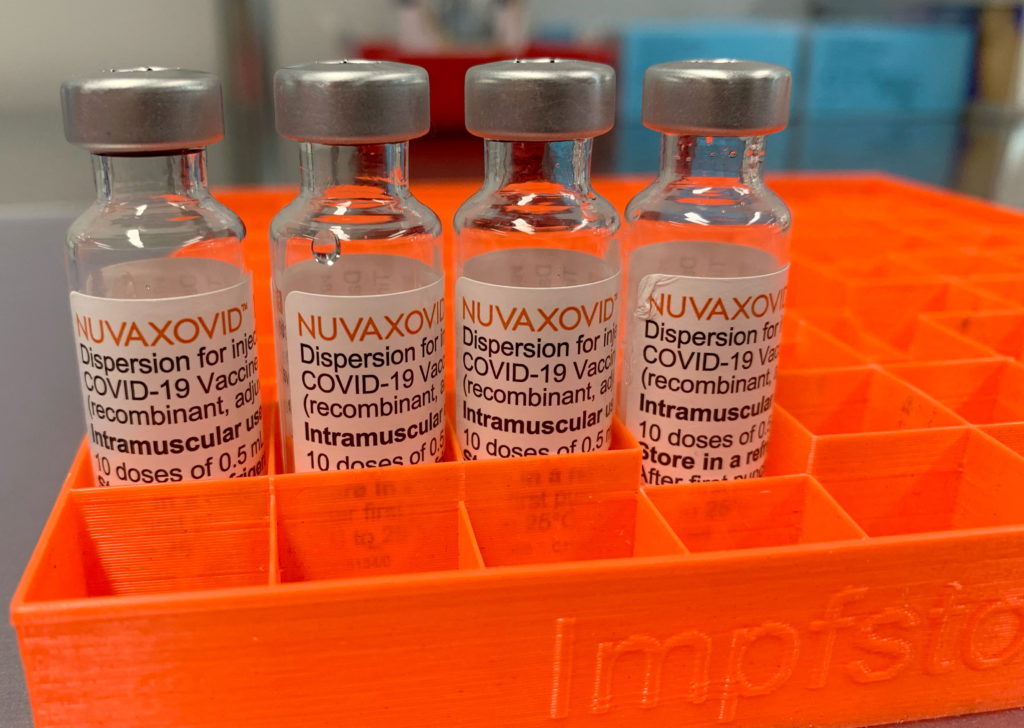
The Novavax COVID-19 vaccine, Nuvaxovid, finally secured conditional FDA approval in July 2023, offering a new option in the fight against the pandemic. However, this approval comes with caveats. While representing a significant step forward, particularly for individuals hesitant about mRNA vaccines, Nuvaxovid's arrival is not without its limitations. This article delves into the details of the approval, highlighting both the benefits and the drawbacks of this protein-subunit vaccine.
What makes Novavax different?
Unlike the Pfizer-BioNTech and Moderna vaccines which utilize mRNA technology, Novavax's Nuvaxovid employs a more traditional protein-subunit approach. This method uses purified fragments of the virus's spike protein to trigger an immune response. This difference has been a key factor driving interest in the vaccine, especially amongst those wary of the newer mRNA technology. Many individuals have expressed concerns about the speed of mRNA vaccine development, and the established track record of protein-subunit vaccines offers a degree of comfort.
The FDA Approval and its Conditions:
The FDA's conditional approval signifies that Nuvaxovid meets the agency's safety and efficacy standards for preventing COVID-19. However, the term "conditional" is crucial. This means the approval is contingent upon ongoing monitoring of the vaccine's long-term safety and effectiveness. Novavax is required to continue submitting data to the FDA to confirm the vaccine's continued benefit and safety profile. This is standard practice for new vaccines and is not necessarily a cause for alarm.
Limitations of the Novavax Vaccine:
While offering a valuable alternative, Nuvaxovid has certain limitations:
-
Efficacy: Clinical trials demonstrated a slightly lower efficacy rate compared to the mRNA vaccines, particularly against newer variants. While still effective in preventing severe illness and hospitalization, its overall protection might be less robust. [Link to relevant FDA data/clinical trial results]
-
Logistics and Distribution: The vaccine requires specific storage and handling conditions, potentially presenting logistical challenges compared to mRNA vaccines which can be stored at higher temperatures for longer periods. This could impact its accessibility in certain regions or healthcare settings.
-
Market Competition: The demand for COVID-19 vaccines has significantly decreased since the initial surge. With other readily available options, Nuvaxovid faces a competitive market environment.
Who should consider the Novavax vaccine?
The Novavax vaccine presents a viable option for individuals who:
- Have concerns about mRNA technology.
- Prefer a more traditionally developed vaccine.
- Have experienced side effects from other COVID-19 vaccines.
However, it's crucial to consult with a healthcare professional to determine if Nuvaxovid is the right choice based on individual health conditions and risk factors.
Looking Ahead:
The conditional approval of the Novavax COVID-19 vaccine expands the available options for vaccination against COVID-19, which remains an ongoing public health concern. The continued monitoring of its long-term efficacy and safety will be crucial in determining its long-term role in the pandemic response. Further research into the vaccine’s effectiveness against emerging variants is also essential. Staying informed about updates from the FDA and the CDC remains paramount.
Call to Action: Consult your doctor to discuss which COVID-19 vaccine is best suited for your individual needs. For more information, visit the CDC website [link to CDC website] and the FDA website [link to FDA website].

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval: The Novavax COVID-19 Vaccine And Its Limitations. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Beyond The Game Exploring The Key Differences In Joel And Ellies Relationship In The Last Of Us Season 2
May 20, 2025
Beyond The Game Exploring The Key Differences In Joel And Ellies Relationship In The Last Of Us Season 2
May 20, 2025 -
 Teaser Talk Unraveling The Story Behind Ntrs Global Travels
May 20, 2025
Teaser Talk Unraveling The Story Behind Ntrs Global Travels
May 20, 2025 -
 Hrithik Roshan Jr Ntr And Kiara Advani In War 2s Explosive First Look
May 20, 2025
Hrithik Roshan Jr Ntr And Kiara Advani In War 2s Explosive First Look
May 20, 2025 -
 Ethereum Investment Surge 200 Million Inflows After Shanghai Upgrade
May 20, 2025
Ethereum Investment Surge 200 Million Inflows After Shanghai Upgrade
May 20, 2025 -
 Repeated Backlash Lizzo On The Challenges And Controversies Of Her Career
May 20, 2025
Repeated Backlash Lizzo On The Challenges And Controversies Of Her Career
May 20, 2025
