Conditional FDA Approval: Novavax COVID-19 Vaccine Faces Usage Limits
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Conditional FDA Approval: Novavax COVID-19 Vaccine Faces Usage Limits
The FDA's conditional approval of the Novavax COVID-19 vaccine marks a significant step, but with limitations. While offering an alternative for those hesitant about mRNA vaccines, its restricted usage raises questions about its broader impact on the pandemic response. This article delves into the details of the approval, the reasons behind the limitations, and what this means for public health.
Novavax's Nuances: Why the Conditional Approval?
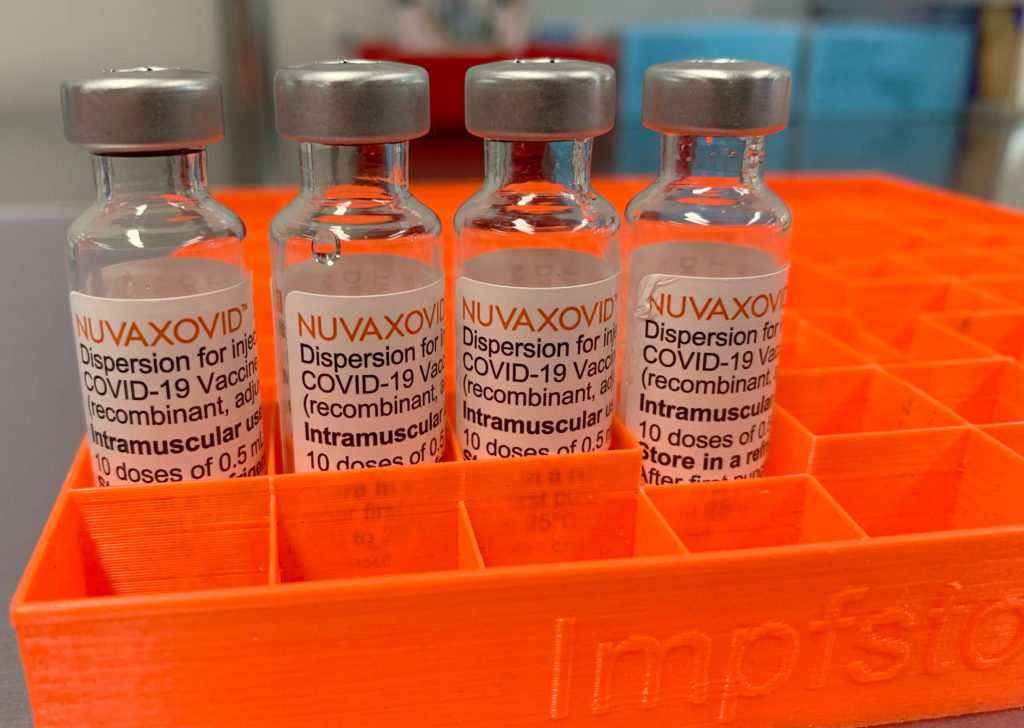
The Novavax vaccine, marketed as Nuvaxovid, uses a different technology than the widely deployed Pfizer-BioNTech and Moderna vaccines. Instead of mRNA, it employs a protein subunit technology, a more traditional approach. This difference has fueled hope among vaccine-hesitant individuals seeking an alternative. However, the FDA's conditional approval reflects several factors influencing its restricted usage:
- Lower Efficacy: Clinical trials demonstrated lower efficacy compared to mRNA vaccines, particularly against newer variants. This lower efficacy, while still offering protection, necessitated a more cautious approach to its rollout.
- Limited Data: The FDA's approval is based on a smaller dataset compared to the vast amount of data accumulated for mRNA vaccines. Further research is needed to fully understand its long-term efficacy and safety profile.
- Production Challenges: Novavax faced production bottlenecks, delaying its widespread availability. This limited supply impacted the initial rollout and contributed to the targeted approach in its approval.
Who Can Receive the Novavax Vaccine?
Currently, the Novavax vaccine is authorized for use in individuals 18 years of age and older. This age restriction reflects the limited data available for younger populations. Furthermore, its use is largely contingent on individual preference and the availability of the vaccine in specific locations. The FDA’s conditional approval emphasizes that the vaccine is an option, not a mandatory replacement for existing vaccines.
Addressing Concerns about Vaccine Hesitancy:
The introduction of the Novavax vaccine offers a potential avenue to address vaccine hesitancy. Many individuals hesitant about mRNA technology may find the protein subunit approach more acceptable. However, it’s crucial to dispel misinformation and provide accurate information about both the benefits and limitations of the Novavax vaccine. Public health campaigns focusing on transparent communication are crucial to ensure the safe and effective deployment of this new vaccine option.
The Road Ahead: Ongoing Monitoring and Research
The FDA’s conditional approval underscores the ongoing need for continued monitoring and research. Post-market surveillance will be critical to track the vaccine's long-term efficacy, safety, and its overall impact on public health. Further clinical trials are also planned to expand the data on different age groups and explore its effectiveness against emerging variants.
Conclusion: A Step Forward, But with Cautions
The conditional approval of the Novavax COVID-19 vaccine represents a step forward in providing diverse vaccine options. However, its restricted usage highlights the importance of considering efficacy, safety data, and production capacity when evaluating new vaccines. As more data emerges and production increases, the role of the Novavax vaccine in combating the pandemic may become clearer. For up-to-date information and guidance, always consult your healthcare provider or refer to reputable sources like the and the .

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval: Novavax COVID-19 Vaccine Faces Usage Limits. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Trump Era Decision Supreme Court Greenlights End To Tps For Some Venezuelans
May 20, 2025
Trump Era Decision Supreme Court Greenlights End To Tps For Some Venezuelans
May 20, 2025 -
 Record Bitcoin Etf Investments 5 B Inflow Signals Market Confidence
May 20, 2025
Record Bitcoin Etf Investments 5 B Inflow Signals Market Confidence
May 20, 2025 -
 Enhanced Data Center Performance Qualcomm Launches Nvidia Compatible Cpus
May 20, 2025
Enhanced Data Center Performance Qualcomm Launches Nvidia Compatible Cpus
May 20, 2025 -
 Trumps Call For Immediate Russia Ukraine Truce Negotiations
May 20, 2025
Trumps Call For Immediate Russia Ukraine Truce Negotiations
May 20, 2025 -
 Big Changes Coming To Peaky Blinders New Series Officially Announced
May 20, 2025
Big Changes Coming To Peaky Blinders New Series Officially Announced
May 20, 2025
