Conditional FDA Approval For Novavax COVID-19 Vaccine: What It Means
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
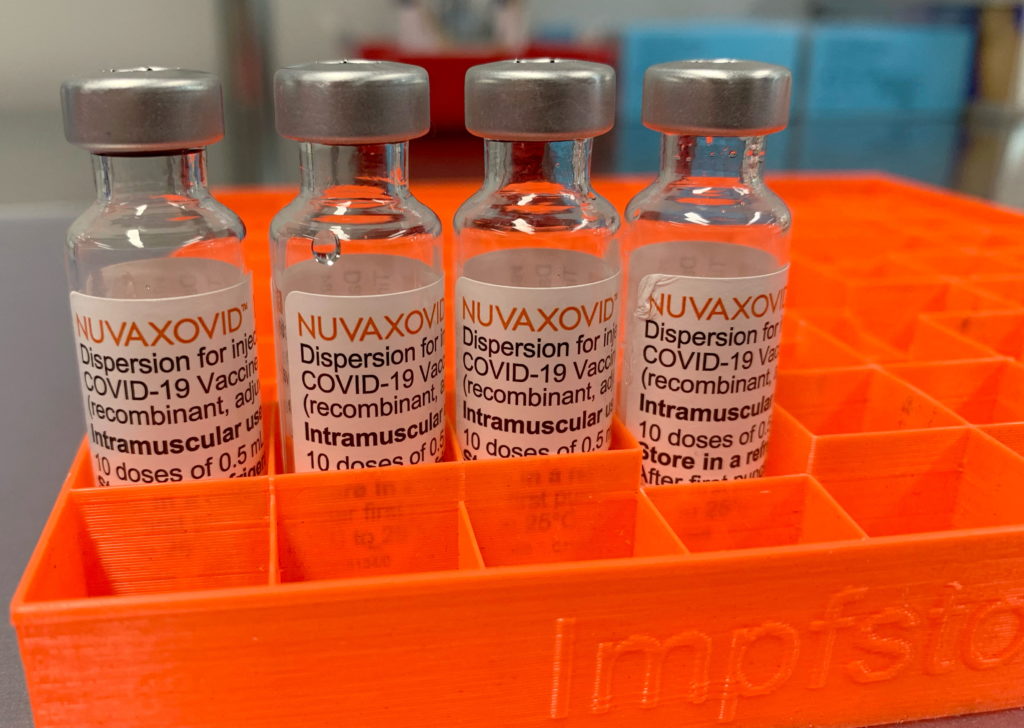
Conditional FDA Approval for Novavax COVID-19 Vaccine: What it Means for You
The arrival of a new COVID-19 vaccine is always significant news, and the recent conditional FDA approval of the Novavax vaccine is no exception. This approval marks a crucial step in the ongoing fight against the pandemic, offering a different vaccine option for those who may have hesitated to receive mRNA vaccines like Pfizer-BioNTech or Moderna. But what does this conditional approval really mean, and how does it affect you? Let's delve into the details.
Understanding Conditional Approval
The FDA's conditional approval, also known as Emergency Use Authorization (EUA) in this context, isn't the same as a full licensure. It means the agency has determined that the benefits of the Novavax vaccine outweigh its known and potential risks, based on available data. However, further studies and data collection are still required to solidify its long-term safety and efficacy profile. This is a common pathway for new vaccines and medications, allowing quicker access to potentially life-saving treatments while ongoing research continues.
What Makes Novavax Different?
Unlike the mRNA vaccines, Novavax's Nuvaxovid uses a more traditional protein subunit technology. This technology has been used for decades in other vaccines, such as the hepatitis B vaccine. This traditional approach might appeal to individuals hesitant about mRNA vaccines due to concerns about their novel technology. This difference could significantly impact vaccine uptake among hesitant populations.
Key Features of the Novavax Vaccine:
- Protein Subunit Technology: Utilizes a harmless piece of the virus (the spike protein) to trigger an immune response.
- Two-Dose Regimen: Similar to many traditional vaccines, Nuvaxovid requires two doses administered several weeks apart.
- Storage Requirements: Generally requires standard refrigeration, making distribution potentially easier in some settings compared to mRNA vaccines.
- Efficacy: Clinical trials demonstrated high efficacy against COVID-19, although this efficacy may vary against different variants.
Who Should Consider the Novavax Vaccine?
The Novavax vaccine offers a valuable alternative for several groups:
- Individuals hesitant about mRNA vaccines: The different technology might alleviate concerns about mRNA-based vaccines.
- People with specific medical conditions: While not a replacement for consulting a doctor, those with concerns about mRNA vaccines due to pre-existing conditions should discuss this option with their healthcare provider.
- Those seeking a different approach: Some individuals may simply prefer a different vaccine type based on personal preference.
Potential Side Effects:
Like all vaccines, Novavax can cause side effects. Common side effects include pain at the injection site, fatigue, headache, muscle aches, and nausea. These are generally mild and short-lived. Serious side effects are rare. It's crucial to report any concerning side effects to your healthcare provider.
Looking Ahead: Continued Monitoring and Full Approval
While conditional approval is a significant step, Novavax will continue to be monitored closely by the FDA. Further studies will evaluate its long-term safety and effectiveness against emerging variants. The ultimate goal is full FDA licensure, providing even greater confidence in the vaccine's safety and efficacy.
Conclusion:
The conditional FDA approval of the Novavax COVID-19 vaccine offers a welcome addition to the existing vaccine arsenal. Its different technology and potential to reach vaccine-hesitant populations represent a significant step in the global fight against COVID-19. Remember to consult with your healthcare provider to determine the best vaccine choice for your individual circumstances. Staying informed about vaccine developments is crucial in protecting yourself and your community.
Keywords: Novavax, COVID-19 Vaccine, FDA Approval, Conditional Approval, Nuvaxovid, mRNA Vaccine, Protein Subunit Vaccine, Vaccine Hesitancy, Vaccine Safety, Vaccine Efficacy, COVID-19 Pandemic, Public Health.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Conditional FDA Approval For Novavax COVID-19 Vaccine: What It Means. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Moodys Downgrade Fails To Dampen Market Strong Gains For S And P 500 Dow And Nasdaq
May 20, 2025
Moodys Downgrade Fails To Dampen Market Strong Gains For S And P 500 Dow And Nasdaq
May 20, 2025 -
 Fans React To Jon Jones Cryptic I M Done Tweet Aspinall Bout In Limbo
May 20, 2025
Fans React To Jon Jones Cryptic I M Done Tweet Aspinall Bout In Limbo
May 20, 2025 -
 Moodys Downgrade Unfazed S And P 500 Dow And Nasdaq Post Strong Gains
May 20, 2025
Moodys Downgrade Unfazed S And P 500 Dow And Nasdaq Post Strong Gains
May 20, 2025 -
 Stock Market Update S And P 500 Dow And Nasdaq Rise Amidst Moodys Credit Rating Action
May 20, 2025
Stock Market Update S And P 500 Dow And Nasdaq Rise Amidst Moodys Credit Rating Action
May 20, 2025 -
 Ufc Fans Erupt Jon Jones Controversial Aspinall Remarks Explained
May 20, 2025
Ufc Fans Erupt Jon Jones Controversial Aspinall Remarks Explained
May 20, 2025
