Novavax's COVID-19 Vaccine: FDA Approval And Usage Restrictions Explained
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
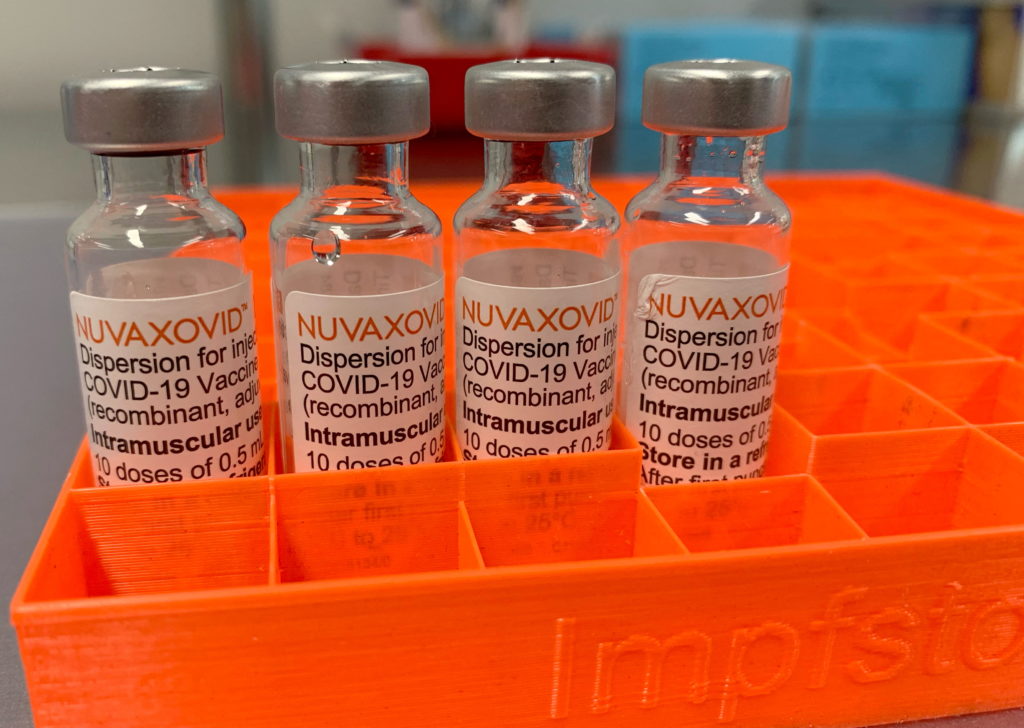
Novavax's COVID-19 Vaccine: FDA Approval and Usage Restrictions Explained
The arrival of Novavax's COVID-19 vaccine, Nuvaxovid (also known as Covovax internationally), marked a significant development in the global fight against the pandemic. Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, Novavax utilizes a more traditional protein subunit technology. This difference has led to considerable interest, but also questions surrounding its FDA approval and usage restrictions. Let's delve into the specifics.
FDA Approval and Emergency Use Authorization (EUA):
Novavax received Emergency Use Authorization (EUA) from the FDA in July 2022 for individuals 18 years of age and older. This EUA, crucial for its initial rollout, paved the way for wider distribution. While it initially lacked full FDA approval, the path to full licensure was ultimately cleared, signifying a rigorous review process and validation of the vaccine's safety and efficacy profile. The FDA's decision underscored the importance of diverse vaccine technologies in combating the virus. This contrasts with the relatively swift EUA approvals granted to mRNA vaccines earlier in the pandemic.
Understanding the Vaccine's Mechanism:
Unlike mRNA vaccines that instruct cells to produce the virus's spike protein, Novavax's vaccine utilizes a recombinant nanoparticle technology. This means that it uses harmless pieces of the virus's spike protein to stimulate an immune response. This more traditional approach might appeal to individuals hesitant about mRNA technology, although it’s important to emphasize that both technologies have proven safe and effective. The protein subunit approach has been used for decades in other vaccines, offering a familiar framework for some.
Usage Restrictions and Considerations:
While generally well-tolerated, Nuvaxovid does have some usage restrictions:
- Age: The initial EUA, and subsequent full approval, specified a minimum age of 18. Further studies may expand its use to younger populations.
- Allergies: As with any vaccine, individuals with known severe allergic reactions to any of its components should consult their physician before receiving the shot. Common allergic reactions to vaccines are rare but should be carefully monitored.
- Pre-existing Conditions: Individuals with pre-existing conditions should discuss vaccination with their healthcare provider to assess potential risks and benefits.
- Pregnancy and Breastfeeding: Pregnant or breastfeeding individuals should consult their doctor for personalized guidance.
Efficacy and Safety Profile:
Clinical trials demonstrated that Novavax's vaccine is highly effective in preventing symptomatic COVID-19. While its efficacy might vary slightly compared to other vaccines, its safety profile is considered comparable. Post-market surveillance continues to monitor its long-term effects and efficacy against emerging variants. The FDA's ongoing monitoring and data analysis are vital for ensuring public safety.
Conclusion: Novavax's Role in the COVID-19 Vaccination Landscape:
Novavax's COVID-19 vaccine offers a valuable addition to the existing arsenal of vaccines. Its different mechanism of action, along with its FDA approval and established safety profile, provides an alternative for individuals who may have preferred a non-mRNA vaccine. However, it is crucial to consult with a healthcare professional to determine the most suitable vaccine based on individual health circumstances. Staying informed about vaccine updates and guidelines remains vital in navigating the ongoing pandemic.
Keywords: Novavax, COVID-19 vaccine, Nuvaxovid, Covovax, FDA approval, EUA, protein subunit vaccine, mRNA vaccine, vaccine safety, vaccine efficacy, usage restrictions, COVID-19 vaccination, pandemic, vaccine technology.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax's COVID-19 Vaccine: FDA Approval And Usage Restrictions Explained. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Uzis New Ride Mercedes Benz Gifts Electric G Class To League Of Legends Icon
May 21, 2025
Uzis New Ride Mercedes Benz Gifts Electric G Class To League Of Legends Icon
May 21, 2025 -
 Juvenile Delinquency Two Boys Charged With Church Break In And Defecation
May 21, 2025
Juvenile Delinquency Two Boys Charged With Church Break In And Defecation
May 21, 2025 -
 A J Perez On Brett Favre Threats Untolds Future And The Favre Scandal
May 21, 2025
A J Perez On Brett Favre Threats Untolds Future And The Favre Scandal
May 21, 2025 -
 Assassins Creed Shadows Why Ubisoft Restrict Animal Killing
May 21, 2025
Assassins Creed Shadows Why Ubisoft Restrict Animal Killing
May 21, 2025 -
 The Making Of Untold Brett Favre A J Perez On Facing Pressure And Production Issues
May 21, 2025
The Making Of Untold Brett Favre A J Perez On Facing Pressure And Production Issues
May 21, 2025
