Novavax Vaccine Gets FDA Nod, But With Uncommon Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax Vaccine Gets FDA Nod, But with Uncommon Usage Restrictions
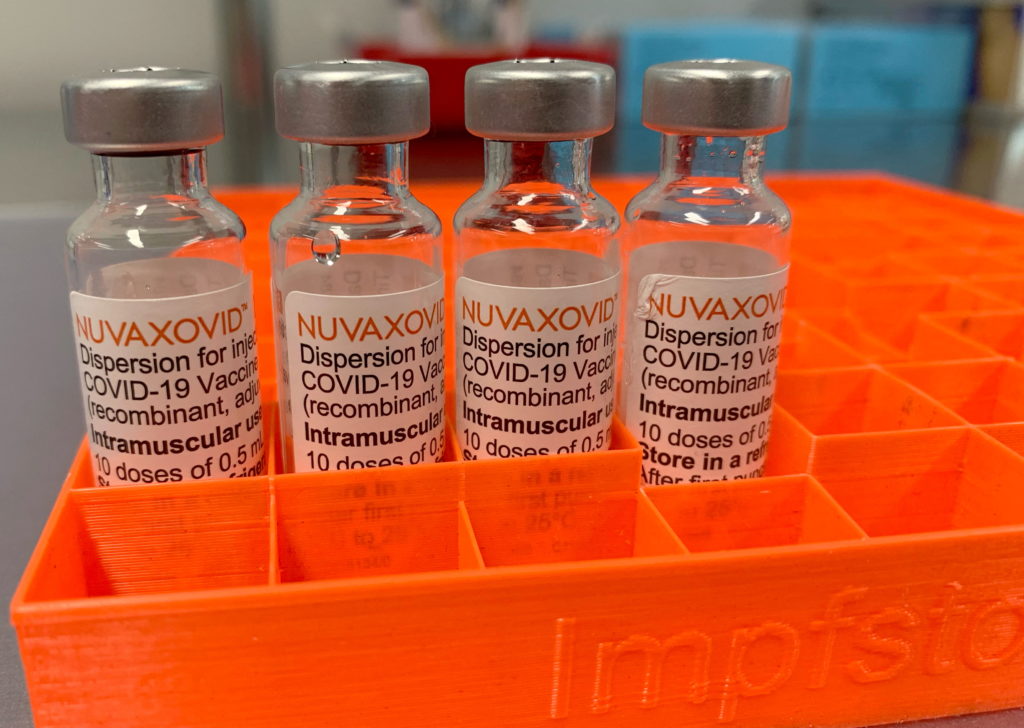
The FDA has finally approved the Novavax COVID-19 vaccine, but its rollout will be significantly hampered by unusual limitations. This landmark decision, announced [Insert Date], comes after months of delay and raises questions about the vaccine's future market share. While hailed as a victory for vaccine diversity, the stringent restrictions placed on its use cast a shadow over its potential impact on the ongoing pandemic.
The approval applies to adults 18 years and older, but the FDA's stipulations set it apart from other authorized COVID-19 vaccines. Unlike Pfizer-BioNTech and Moderna's mRNA vaccines, or Johnson & Johnson's viral vector vaccine, Novavax's protein-subunit approach is now relegated to a more specific niche. This is due primarily to the emergence of newer variants and the established dominance of the existing vaccines.
Why the Restrictions? A Look at the FDA's Decision
The FDA's decision to approve Novavax, while seemingly positive, is tempered by several key factors:
- Lower Efficacy: Clinical trial data showed Novavax's efficacy against earlier COVID-19 variants to be lower than other authorized vaccines. While effective, it's not as effective against currently circulating strains.
- Limited Demand: With high vaccination rates achieved through existing vaccines, the demand for a new vaccine with limitations is expected to be significantly lower. This could impact the vaccine's overall cost-effectiveness.
- Logistical Challenges: The vaccine's two-dose regimen, requiring specific storage and handling conditions, presents additional logistical hurdles for distribution, particularly in underserved areas.
The FDA's press release highlighted the vaccine's potential benefit for individuals hesitant to receive mRNA vaccines, citing its conventional technology as a possible bridge to vaccine acceptance for this population. However, the efficacy concerns raise questions about how effective this strategy will prove to be in the long run. [Insert Link to FDA Press Release].
What Does This Mean for the Future of COVID-19 Vaccination?
The Novavax approval presents a complex scenario. While offering another option, the restrictive usage guidelines significantly limit its potential impact. Experts are debating the vaccine's role in boosting overall vaccination rates and its effectiveness against emerging variants. Further research is crucial to determine its long-term efficacy and potential applications.
Several questions remain unanswered:
- Will the limited use case impact the uptake of the vaccine?
- Will Novavax be effective against future COVID-19 variants?
- What will be the long-term cost-benefit analysis of this vaccine given the restrictions?
The approval highlights the dynamic nature of vaccine development and the ongoing need for adaptable strategies in the fight against infectious diseases. Further monitoring of the vaccine's performance in real-world settings is necessary to fully assess its contribution to public health. This situation serves as a reminder that even with FDA approval, the complexities surrounding vaccine deployment and public health strategies continue to evolve.
Call to Action: Stay informed about the latest COVID-19 vaccine updates from trusted sources like the CDC and WHO. Consult your healthcare provider to determine the best vaccination strategy for you. [Insert Links to CDC and WHO websites].

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax Vaccine Gets FDA Nod, But With Uncommon Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Ubisoft Milan Expands Team For Aaa Rayman Title
May 21, 2025
Ubisoft Milan Expands Team For Aaa Rayman Title
May 21, 2025 -
 Rain And Falling Temperatures Your Week Ahead Weather Outlook
May 21, 2025
Rain And Falling Temperatures Your Week Ahead Weather Outlook
May 21, 2025 -
 Overnight Storms And Temperature Plunge Forecast For Charlotte Area
May 21, 2025
Overnight Storms And Temperature Plunge Forecast For Charlotte Area
May 21, 2025 -
 Japanese Businesses And Nature Conservation 160 Companies Compete For Enhanced Corporate Value
May 21, 2025
Japanese Businesses And Nature Conservation 160 Companies Compete For Enhanced Corporate Value
May 21, 2025 -
 Breaking And Entering Two Boys Charged After Church Bathroom Incident
May 21, 2025
Breaking And Entering Two Boys Charged After Church Bathroom Incident
May 21, 2025
