Novavax COVID-19 Vaccine Receives FDA Approval: Key Restrictions To Consider
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Receives FDA Approval: Key Restrictions to Consider
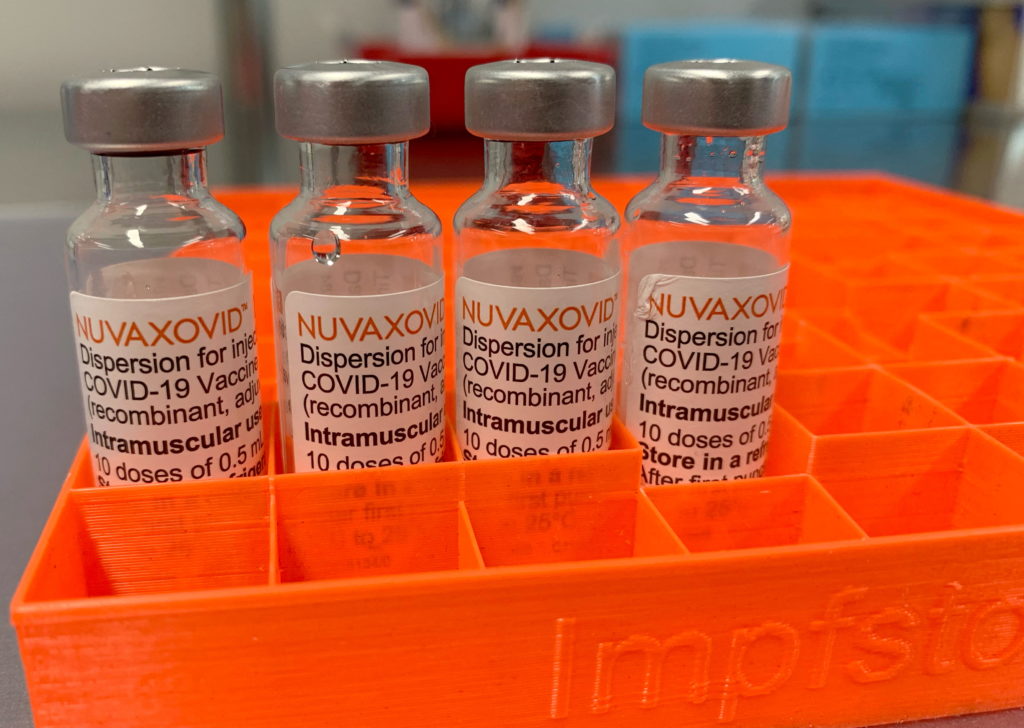
The FDA's approval of the Novavax COVID-19 vaccine, Nuvaxovid, marks a significant milestone in the fight against the pandemic. This protein-subunit vaccine offers a different approach compared to the mRNA vaccines from Pfizer-BioNTech and Moderna, potentially appealing to individuals hesitant about the newer technologies. However, it's crucial to understand the key restrictions surrounding its use. This article will delve into the specifics of the FDA approval and highlight the important limitations to consider.
Understanding the Novavax Vaccine:
Unlike mRNA vaccines that use messenger RNA to instruct cells to produce a viral protein, Novavax utilizes a more traditional approach. Nuvaxovid uses a lab-made version of the spike protein found on the surface of the SARS-CoV-2 virus. This protein is combined with an adjuvant, a substance that boosts the immune response. This method has been used in other vaccines for decades, potentially contributing to greater public trust. This approach may appeal to individuals who prefer a more established vaccine technology.
FDA Approval and its Significance:
The FDA's approval signifies that the vaccine has met rigorous safety and efficacy standards. This contrasts with the previous Emergency Use Authorizations (EUAs) granted to other COVID-19 vaccines. Approval provides a higher level of assurance regarding the vaccine's safety and effectiveness. The approval of Nuvaxovid expands vaccine options, providing more choices for individuals and potentially increasing vaccination rates.
Key Restrictions and Considerations:
While the FDA approval is positive news, several key restrictions should be noted:
-
Age Restrictions: Currently, the Novavax vaccine is approved for individuals 18 years of age and older. Further studies are needed to determine its safety and efficacy in younger populations. Parents and guardians should consult with their pediatricians before considering vaccination for individuals under 18.
-
Allergic Reactions: As with any vaccine, there's a potential for allergic reactions. Individuals with known severe allergies to any component of the vaccine should consult with their doctor before receiving the shot. The FDA recommends close monitoring for allergic reactions after vaccination.
-
Efficacy Compared to Other Vaccines: While effective, studies have shown Novavax's efficacy might be slightly lower compared to mRNA vaccines, particularly against emerging variants. This doesn't necessarily diminish its value, but it's a factor to consider. Staying updated on variant-specific efficacy data is crucial. [Link to CDC website on COVID-19 vaccine efficacy]
-
Storage and Handling: Novavax vaccine requires specific storage and handling conditions, potentially impacting its availability in certain settings compared to mRNA vaccines. This could lead to logistical challenges for distribution in some areas.
-
Limited Data on Long-Term Effects: As with all newly approved vaccines, long-term data on the effects of the Novavax vaccine is still being gathered. Ongoing monitoring is crucial for assessing its long-term safety and effectiveness profile.
Conclusion:
The FDA approval of the Novavax COVID-19 vaccine is a welcome development, offering another tool in the fight against the pandemic. However, it's essential to carefully consider the outlined restrictions and to consult with a healthcare provider to determine if this vaccine is the right choice for you. Staying informed about the latest research and updates is vital in making informed decisions regarding COVID-19 vaccination. Remember to consult your doctor or pharmacist with any questions or concerns. Staying up-to-date on your vaccinations remains a crucial step in protecting yourself and your community.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Receives FDA Approval: Key Restrictions To Consider. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Spains Regulatory Clampdown Airbnbs 66 000 Listing Purge
May 20, 2025
Spains Regulatory Clampdown Airbnbs 66 000 Listing Purge
May 20, 2025 -
 Hong Kongs Cathay Pacific Welcomes First Training Program Graduates
May 20, 2025
Hong Kongs Cathay Pacific Welcomes First Training Program Graduates
May 20, 2025 -
 Jon Jones Ufc Kept Aspinalls Injury A Secret From Fight Fans
May 20, 2025
Jon Jones Ufc Kept Aspinalls Injury A Secret From Fight Fans
May 20, 2025 -
 Balis Tourism Crisis A Need For International Collaboration On Safety And Conduct
May 20, 2025
Balis Tourism Crisis A Need For International Collaboration On Safety And Conduct
May 20, 2025 -
 Amazon And A24 Team Up For A Surprising New Series
May 20, 2025
Amazon And A24 Team Up For A Surprising New Series
May 20, 2025
