Novavax COVID-19 Vaccine Gets FDA Nod, Yet Usage Is Significantly Restricted
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Approved, but with Significant Limitations: What You Need to Know
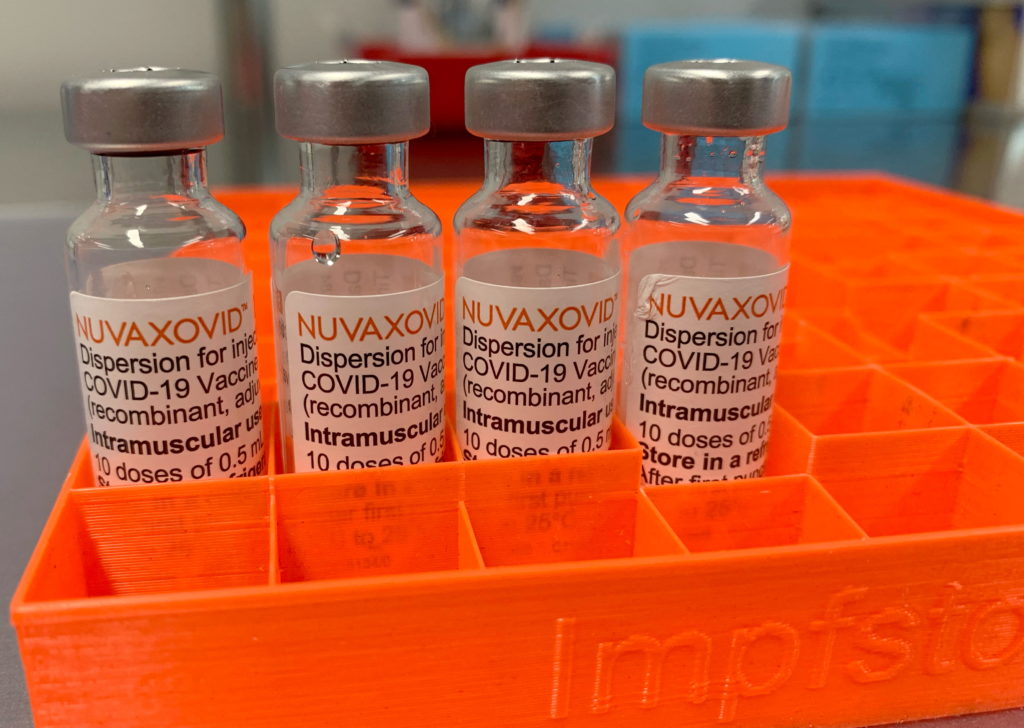
The FDA has finally granted approval to the Novavax COVID-19 vaccine, Nuvaxovid, marking a significant development in the ongoing fight against the pandemic. However, the approval comes with considerable caveats, significantly restricting its usage compared to other authorized vaccines. This raises important questions about the vaccine's role in the current landscape of COVID-19 prevention.
Limited Authorization: A Narrow Path to Approval
While the approval is a victory for Novavax, the FDA's authorization is far from a blanket endorsement. The vaccine is only authorized for individuals 18 years of age and older, leaving a significant portion of the population – particularly adolescents and children – ineligible. This contrasts sharply with the broader authorization granted to mRNA vaccines like Pfizer-BioNTech and Moderna. Furthermore, the FDA's approval emphasizes the vaccine's use primarily as an alternative for individuals who cannot or will not receive an mRNA vaccine, highlighting the limitations in its overall impact.
Why the Restrictions? Understanding the Nuances
The restricted usage stems from several factors. Firstly, clinical trial data for Nuvaxovid, while demonstrating efficacy, didn't match the high efficacy rates seen in the mRNA vaccines. Secondly, the rollout of the mRNA vaccines has already established a high level of vaccination coverage in many countries, reducing the immediate urgency for a new vaccine with limited authorization. This situation contrasts with the earlier stages of the pandemic, where additional vaccine options were desperately needed.
Comparing Novavax to Existing Vaccines: A Key Consideration
The existing landscape of COVID-19 vaccines significantly impacts Nuvaxovid's role. The mRNA vaccines, Pfizer-BioNTech and Moderna, boast higher efficacy rates and have established a broad safety profile through widespread use. They also offer wider age-range authorization, making them the preferred choice for most individuals. Novavax's protein-based technology, while offering a different approach, doesn't currently provide a compelling advantage to outweigh these factors.
What Does This Mean for the Future of COVID-19 Vaccination?
The limited authorization of the Novavax vaccine raises important questions about future vaccine development and distribution. While it offers a choice for those unable or unwilling to receive mRNA vaccines, its restricted use suggests that future focus may shift towards more targeted vaccine approaches, like updated boosters addressing emerging variants. The longer-term significance of Nuvaxovid remains to be seen, particularly as the pandemic evolves and new variants emerge. The efficacy against emerging variants is also an area requiring continued monitoring and research.
Moving Forward: Informed Choices and Public Health Strategies
The FDA's decision highlights the complexities of vaccine development and deployment. The approval of Novavax's vaccine offers a niche option, but its restricted usage underscores the crucial role of data-driven decision-making and the importance of public health strategies that leverage the most effective available tools. Staying informed about vaccine updates and consulting with healthcare professionals remains crucial for individuals making choices about COVID-19 vaccination.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, vaccine authorization, mRNA vaccines, protein-based vaccine, vaccine efficacy, COVID-19 vaccination, public health, vaccine safety, COVID-19 variants, vaccine rollout.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, Yet Usage Is Significantly Restricted. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Investors Bet Big On Ethereum 200 Million Investment Following Pectra Upgrade
May 21, 2025
Investors Bet Big On Ethereum 200 Million Investment Following Pectra Upgrade
May 21, 2025 -
 J D Vance Challenges Bidens Capacity Following Cancer Announcement
May 21, 2025
J D Vance Challenges Bidens Capacity Following Cancer Announcement
May 21, 2025 -
 Rising Temperatures Rising Risks Examining The Link Between Climate Change And Pregnancy Complications
May 21, 2025
Rising Temperatures Rising Risks Examining The Link Between Climate Change And Pregnancy Complications
May 21, 2025 -
 Cooldown Coming To Charlotte After Overnight Storm Prediction
May 21, 2025
Cooldown Coming To Charlotte After Overnight Storm Prediction
May 21, 2025 -
 Big Budget Rayman Game Ubisoft Milans Open Positions
May 21, 2025
Big Budget Rayman Game Ubisoft Milans Open Positions
May 21, 2025
