Novavax COVID-19 Vaccine Gets FDA Nod, With Uncommon Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
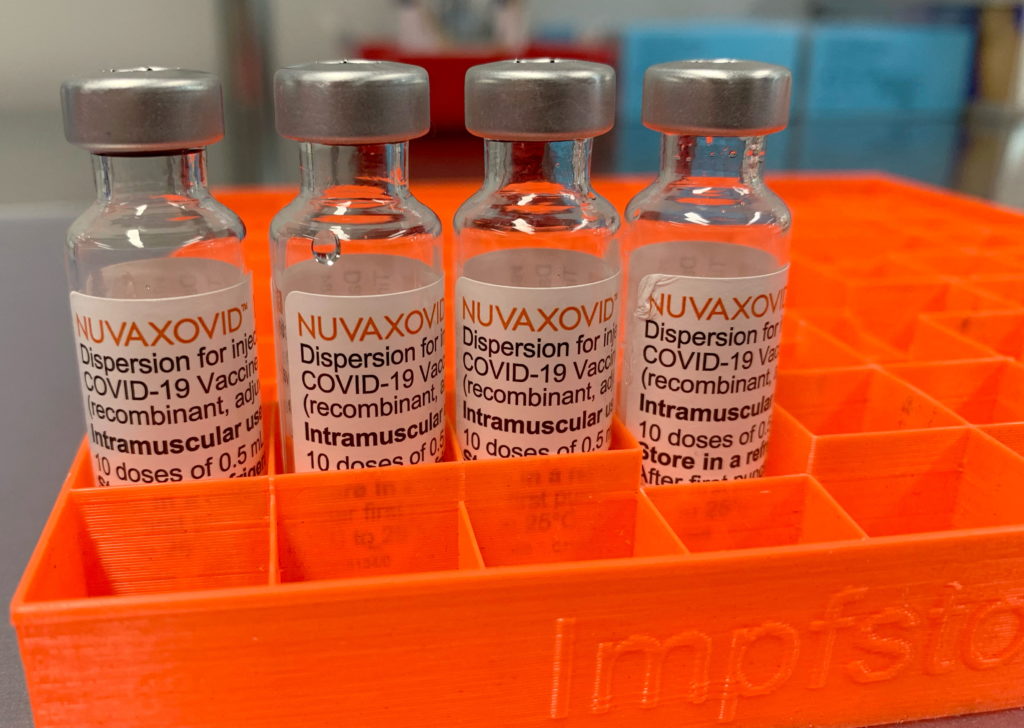
Novavax COVID-19 Vaccine Receives FDA Approval, but with Notable Usage Restrictions
The US Food and Drug Administration (FDA) has granted Emergency Use Authorization (EUA) to the Novavax COVID-19 vaccine, Nuvaxovid, marking a significant development in the nation's fight against the pandemic. However, the approval comes with some uncommon usage restrictions that have sparked discussion amongst medical professionals and public health experts. This article delves into the details of the approval, the specifics of the restrictions, and what they mean for the future of COVID-19 vaccination in the United States.
Understanding Nuvaxovid: A Different Approach
Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, or the viral vector vaccine from Johnson & Johnson, Novavax's Nuvaxovid utilizes a protein subunit technology. This involves using harmless pieces of the virus to stimulate an immune response, a method that some individuals may find more appealing due to its familiarity and established safety profile. This protein-based approach has been a cornerstone of vaccine development for decades, giving it a potentially wider acceptance among vaccine-hesitant populations. However, this traditional approach also has its own set of manufacturing and logistical challenges.
The FDA's Approval and Its Caveats
The FDA's approval is a welcome addition to the existing COVID-19 vaccine arsenal. The agency cited the vaccine's effectiveness and safety profile in its decision. However, the EUA is accompanied by some significant limitations:
- Age Restrictions: The vaccine is currently authorized only for individuals 18 years of age and older. Trials for younger age groups are ongoing, but results are not yet available.
- Limited Supply: Initial supplies of the Novavax vaccine are expected to be limited, potentially impacting widespread rollout and accessibility. This constraint could restrict its immediate impact on overall vaccination rates.
- Two-Dose Regimen: Nuvaxovid requires a two-dose primary vaccination series, administered three weeks apart, mirroring the schedules of other widely-used COVID-19 vaccines.
- Specific Storage Requirements: The vaccine has specific cold chain requirements for storage and transportation, potentially posing challenges for distribution in remote or resource-limited areas.
These restrictions differ from the broader authorizations granted to other COVID-19 vaccines, highlighting the unique aspects of Nuvaxovid's production and deployment.
Implications for the Future of COVID-19 Vaccination
The FDA's approval of the Novavax vaccine offers several potential benefits:
- Increased Vaccine Choice: Offering a different vaccine technology provides an alternative for individuals who may have concerns about mRNA or viral vector vaccines. This increased choice is crucial for boosting overall vaccination rates.
- Potential for Booster Doses: Future studies may explore the use of Nuvaxovid as a booster shot for individuals who have received other COVID-19 vaccines.
- Global Vaccine Equity: Novavax's vaccine production capacity is being utilized globally, potentially contributing to better vaccine distribution in low and middle-income countries.
However, the limited supply and age restrictions initially constrain its immediate impact. The long-term success of the Novavax vaccine will depend on overcoming these logistical hurdles and expanding its availability.
Looking Ahead
The arrival of the Novavax COVID-19 vaccine is a step forward, providing a valuable alternative in the fight against the virus. However, the associated limitations underscore the complexities of vaccine development and distribution. The coming months will be crucial in observing the real-world impact of Nuvaxovid and addressing the challenges associated with its deployment. Further updates on the ongoing trials and expansion of usage guidelines are eagerly awaited by public health officials and the medical community. The effectiveness of the vaccine in various populations and its role in future booster strategies will shape its long-term significance in the pandemic response.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, With Uncommon Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Bitcoin Etf Investments Surge Past 5 Billion What This Means For Investors
May 20, 2025
Bitcoin Etf Investments Surge Past 5 Billion What This Means For Investors
May 20, 2025 -
 Jon Jones Ufc Future Uncertain I M Done Hint Sparks Fan Outrage
May 20, 2025
Jon Jones Ufc Future Uncertain I M Done Hint Sparks Fan Outrage
May 20, 2025 -
 Refinancing Your Mortgage See Current Rates May 19 2025
May 20, 2025
Refinancing Your Mortgage See Current Rates May 19 2025
May 20, 2025 -
 World Prides Washington D C Event A Show Of Strength Amid Political Tensions
May 20, 2025
World Prides Washington D C Event A Show Of Strength Amid Political Tensions
May 20, 2025 -
 Freaky Friday Reunion Jamie Lee Curtis Reveals Current Status With Lindsay Lohan
May 20, 2025
Freaky Friday Reunion Jamie Lee Curtis Reveals Current Status With Lindsay Lohan
May 20, 2025
