Novavax COVID-19 Vaccine Gets FDA Nod, Use Significantly Curtailed
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
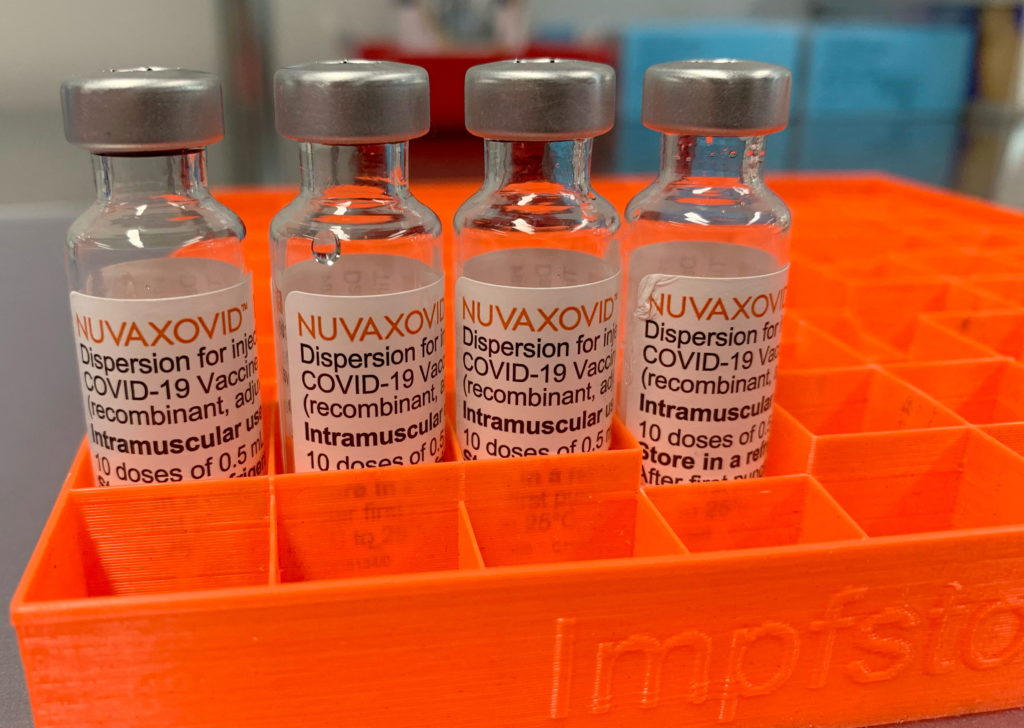
Novavax COVID-19 Vaccine Gets FDA Nod, but Use Significantly Curtailed
The Novavax COVID-19 vaccine, Nuvaxovid, has finally received full approval from the U.S. Food and Drug Administration (FDA), marking a significant milestone for the company. However, the celebration is tempered by the FDA's decision to significantly limit its use, raising questions about the vaccine's future role in the ongoing pandemic. This approval comes after years of development and amidst a dramatically shifted landscape in the COVID-19 vaccine market.
A Long Road to Approval:
Novavax's Nuvaxovid, a protein-subunit vaccine, represents a different technological approach compared to the mRNA vaccines from Pfizer-BioNTech and Moderna, and the viral vector vaccine from Johnson & Johnson. This protein-based approach has been touted for its traditional methodology and potential appeal to vaccine-hesitant individuals. However, the path to FDA approval was fraught with delays and setbacks, impacting its market entry significantly. The FDA's review process, while ultimately successful, highlights the rigorous standards applied to all COVID-19 vaccines.
Limited Authorization: Why the Curtailed Use?
While the full approval is a positive step for Novavax, the FDA's decision to restrict its use is noteworthy. The agency has authorized the vaccine only for individuals 18 years of age and older. This limited authorization contrasts sharply with the broader use of other COVID-19 vaccines. Furthermore, the FDA's statement emphasizes that Nuvaxovid should only be used when other authorized or recommended COVID-19 vaccines are not accessible or clinically appropriate.
Several factors likely contributed to this limited approval:
- Lower Demand: The current demand for COVID-19 vaccines has significantly decreased in the U.S. compared to the peak of the pandemic. This reduced need makes it less likely that Nuvaxovid will be widely used.
- Efficacy Data: While the vaccine showed efficacy against earlier COVID-19 variants, the emergence of newer variants might have impacted its overall effectiveness. Further research into the vaccine's efficacy against current variants is likely needed.
- Market Competition: The existing COVID-19 vaccines already hold a significant market share, making it challenging for a new entrant like Novavax to gain substantial traction.
What Does This Mean for the Future of Nuvaxovid?
The FDA's approval, though limited, provides Novavax with a crucial regulatory endorsement. However, the restricted use raises concerns about the vaccine's market viability. The company will need to aggressively pursue strategies to increase vaccine uptake, potentially focusing on specific demographics or regions where other vaccines are less accessible. Further research and data demonstrating effectiveness against newer variants will be crucial to expand its authorized use. The long-term impact on Novavax's financial performance and the vaccine's role in global vaccination efforts remains uncertain.
Looking Ahead: The Evolving Landscape of COVID-19 Vaccination:
The COVID-19 pandemic continues to evolve, necessitating ongoing vigilance and adaptation in vaccination strategies. While the FDA's approval of Nuvaxovid offers another option, its limited use highlights the dynamic nature of vaccine development and deployment. The future of COVID-19 vaccination will likely involve ongoing research into updated vaccines to combat emerging variants and a continued effort to ensure equitable vaccine access globally. For more information on COVID-19 vaccines and vaccination strategies, consult the .
Keywords: Novavax, COVID-19 vaccine, Nuvaxovid, FDA approval, vaccine efficacy, protein-subunit vaccine, pandemic, vaccination, COVID-19 variants, vaccine hesitancy, public health.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, Use Significantly Curtailed. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Market Rally Continues S And P 500s Six Day Winning Streak And Positive Market Sentiment
May 20, 2025
Market Rally Continues S And P 500s Six Day Winning Streak And Positive Market Sentiment
May 20, 2025 -
 65 000 Airbnb Rentals In Spain Blocked Government Targets Illegal Listings
May 20, 2025
65 000 Airbnb Rentals In Spain Blocked Government Targets Illegal Listings
May 20, 2025 -
 Post Shanghai Upgrade Investors Pour 200 M Into Ethereum Funds
May 20, 2025
Post Shanghai Upgrade Investors Pour 200 M Into Ethereum Funds
May 20, 2025 -
 Market Update S And P 500 Dow And Nasdaq Rise Amidst Moodys Negative Outlook
May 20, 2025
Market Update S And P 500 Dow And Nasdaq Rise Amidst Moodys Negative Outlook
May 20, 2025 -
 Los Angeles Police Department Involved In Major Motorcyclist Pursuit
May 20, 2025
Los Angeles Police Department Involved In Major Motorcyclist Pursuit
May 20, 2025
