Novavax COVID-19 Vaccine Gets FDA Nod, Subject To Specific Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Receives FDA Approval, But With Conditions
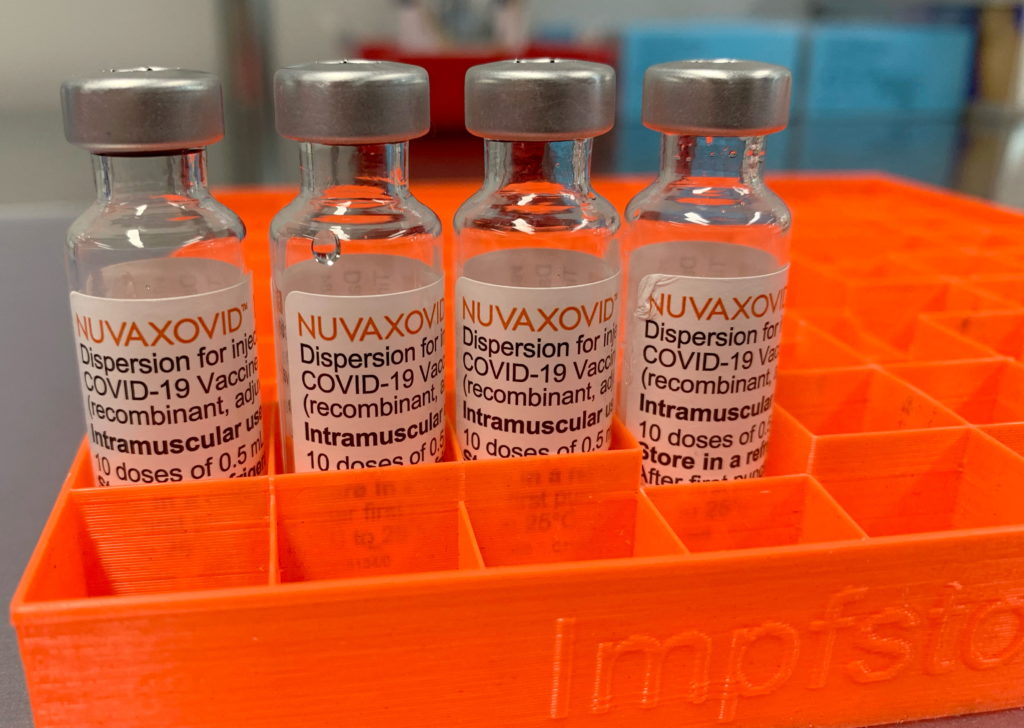
Novavax's COVID-19 vaccine, Nuvaxovid, has finally received Emergency Use Authorization (EUA) from the FDA, marking a significant development in the fight against the pandemic. However, the approval comes with specific restrictions, limiting its immediate impact.
The long-awaited approval of Nuvaxovid offers a new option for individuals seeking a COVID-19 vaccine. Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, or the viral vector vaccine from Johnson & Johnson, Novavax's vaccine uses a more traditional protein subunit technology. This approach might appeal to individuals hesitant about the newer vaccine technologies. The FDA's decision is based on extensive clinical trial data demonstrating the vaccine's safety and efficacy against COVID-19.
What are the Restrictions on Nuvaxovid's Use?
While the FDA's approval is a positive step, it's crucial to understand the limitations. The authorization is specifically for individuals aged 18 years and older. Furthermore, the FDA has placed restrictions on its use, primarily due to concerns about its efficacy against certain variants and its potential side effects. These restrictions may affect the vaccine's widespread rollout and accessibility.
- Limited Efficacy Against Variants: Clinical trial data shows that Nuvaxovid's effectiveness may be lower against certain emerging COVID-19 variants, compared to the mRNA vaccines. This necessitates ongoing monitoring and potential adjustments to the vaccine formula in the future.
- Side Effect Profile: While generally well-tolerated, Nuvaxovid has shown some side effects similar to other vaccines, including pain at the injection site, fatigue, headache, and muscle aches. The FDA will continue to monitor reports of adverse events.
- Dosage and Administration: The vaccine requires two doses administered several weeks apart. Strict adherence to the recommended dosage and administration schedule is crucial for optimal effectiveness.
What Does This Mean for the Future of COVID-19 Vaccination?
The FDA's approval of Nuvaxovid, despite the restrictions, adds another weapon to our arsenal in the fight against COVID-19. It offers an alternative for individuals who may have been hesitant to receive other vaccines due to concerns about their technology. The availability of different vaccine types is vital for ensuring broad vaccination coverage and achieving herd immunity.
However, the limitations highlight the ongoing challenges in developing and deploying effective vaccines against a rapidly evolving virus. The FDA's continued monitoring and potential adjustments to the vaccine's usage guidelines are crucial for optimizing its effectiveness and safety.
Where Can I Learn More?
For more information on the Novavax COVID-19 vaccine, including detailed information on its efficacy, safety profile, and administration, you can consult the following resources:
- The FDA Website: (Replace with actual FDA link for Novavax vaccine information)
- The CDC Website: (Replace with actual CDC link for Novavax vaccine information)
- Your Healthcare Provider: Consult your doctor or healthcare provider to determine if the Novavax vaccine is right for you.
Disclaimer: This article provides general information and should not be considered medical advice. Always consult with a healthcare professional for any health concerns or before making any decisions related to your health or treatment.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, Subject To Specific Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Years Later Jenn Sterger Reflects On The Brett Favre Scandal And Its Devastating Effects
May 20, 2025
Years Later Jenn Sterger Reflects On The Brett Favre Scandal And Its Devastating Effects
May 20, 2025 -
 U S Treasury Yields Fall As Fed Hints At One Rate Cut In 2025
May 20, 2025
U S Treasury Yields Fall As Fed Hints At One Rate Cut In 2025
May 20, 2025 -
 American Innovation How Medical And Scientific Research Drives National Advancement
May 20, 2025
American Innovation How Medical And Scientific Research Drives National Advancement
May 20, 2025 -
 Stock Market Today Six Day Winning Streak For S And P 500 Dow And Nasdaq Follow Suit
May 20, 2025
Stock Market Today Six Day Winning Streak For S And P 500 Dow And Nasdaq Follow Suit
May 20, 2025 -
 New Streaming Release A Critically Acclaimed Wwi Movie With A Stellar Cast
May 20, 2025
New Streaming Release A Critically Acclaimed Wwi Movie With A Stellar Cast
May 20, 2025
