Novavax COVID-19 Vaccine Gets FDA Nod, Strict Usage Guidelines In Place
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
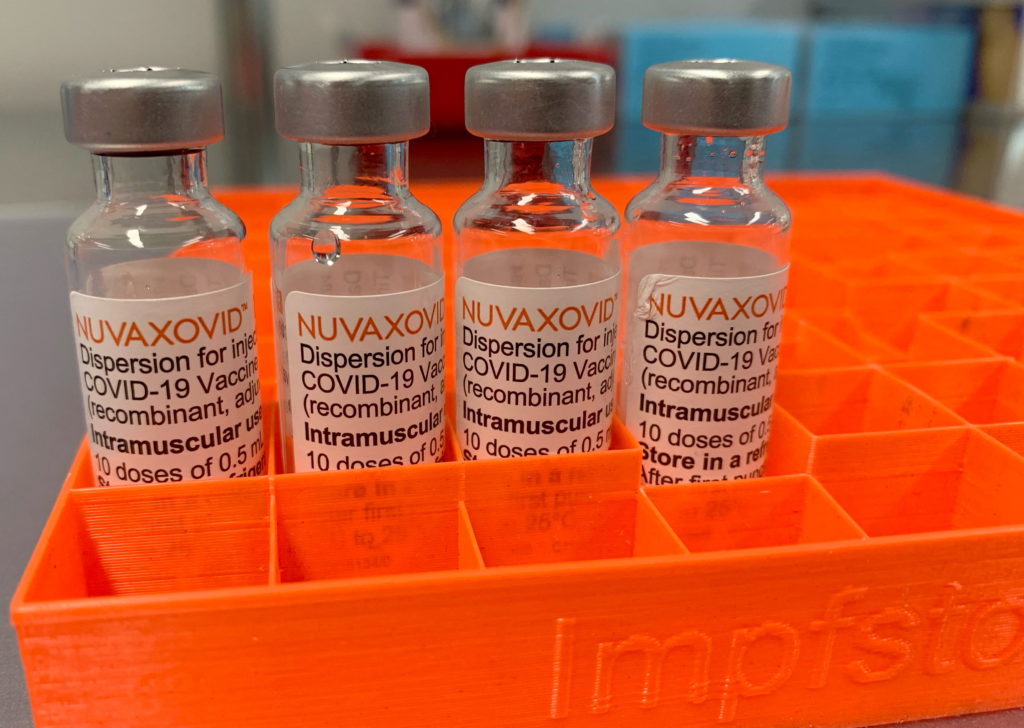
Novavax COVID-19 Vaccine Gets FDA Nod, But with Strict Usage Guidelines
The FDA has finally approved the Novavax COVID-19 vaccine, Nuvaxovid, offering a new option for those hesitant about mRNA technology. However, strict usage guidelines are in place, limiting its immediate impact.
The long-awaited approval of the Novavax COVID-19 vaccine, officially known as Nuvaxovid, by the U.S. Food and Drug Administration (FDA) marks a significant development in the fight against the pandemic. This protein-subunit vaccine offers a different approach compared to the widely used mRNA vaccines from Pfizer-BioNTech and Moderna, potentially appealing to individuals who have expressed concerns about mRNA technology. However, the FDA’s approval comes with caveats, including strict usage guidelines that will limit its immediate rollout and widespread availability.
Why the Delay and Conditional Approval?
The FDA's approval process for the Novavax vaccine has been longer and more scrutinized than for other COVID-19 vaccines. While the vaccine has shown effectiveness in clinical trials, the FDA required extensive data analysis and rigorous testing before granting approval. This rigorous process, while increasing public confidence in vaccine safety, has contributed to the delay. The approval is also conditional, meaning ongoing monitoring of safety and efficacy data is required.
How Does the Novavax Vaccine Work?
Unlike mRNA vaccines that use messenger RNA to instruct the body to produce a viral protein, the Novavax vaccine uses a more traditional approach. It contains a purified version of the spike protein from the SARS-CoV-2 virus. This protein is not infectious but triggers an immune response, teaching the body to recognize and fight the virus. This protein-subunit approach has been used successfully in other vaccines, such as the Hepatitis B vaccine. This different mechanism may make it appealing to those hesitant about the newer mRNA technology.
Strict Usage Guidelines: Who Can Get the Novavax Vaccine?
The FDA's approval comes with strict usage guidelines. Currently, the vaccine is authorized for:
- Individuals 18 years of age and older. There are currently no approved formulations for children and adolescents.
- Use as a primary series: It's not yet approved as a booster shot. Further clinical trials are needed to determine its efficacy as a booster.
This limited authorization means the vaccine won't immediately replace existing vaccines and is unlikely to dramatically alter the current vaccination landscape in the short term.
What This Means for the Future of COVID-19 Vaccination
The Novavax vaccine offers a valuable alternative for individuals who have hesitated to receive an mRNA vaccine. Its approval expands the options available and contributes to a more comprehensive strategy for combatting the pandemic. However, the strict guidelines highlight the importance of continued research and development to refine and improve vaccine technologies.
The FDA's decision underscores the commitment to ensuring vaccine safety and efficacy. While its immediate impact might be limited by the current usage guidelines, the long-term implications for expanding vaccination options and building public trust are significant. The future will likely see further studies evaluating the Novavax vaccine’s use in various populations and as a booster shot.
Call to Action: Consult with your healthcare provider to determine the best COVID-19 vaccination strategy for you. Stay informed about the latest updates on vaccine approvals and guidelines from trusted sources like the and the .

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, Strict Usage Guidelines In Place. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Show Of Unity International Support For President Bidens Cancer Treatment
May 20, 2025
Show Of Unity International Support For President Bidens Cancer Treatment
May 20, 2025 -
 Helldivers 2 May 15th Update New Master Of Ceremony Warbond Rewards
May 20, 2025
Helldivers 2 May 15th Update New Master Of Ceremony Warbond Rewards
May 20, 2025 -
 Immigration And A Reality Tv Show Is Dhs Seriously Considering This
May 20, 2025
Immigration And A Reality Tv Show Is Dhs Seriously Considering This
May 20, 2025 -
 Creator Confirms New Peaky Blinders Series With Significant Shift
May 20, 2025
Creator Confirms New Peaky Blinders Series With Significant Shift
May 20, 2025 -
 Incoming Solar Flares Nasas Urgent Warning Of Potential Power Outages
May 20, 2025
Incoming Solar Flares Nasas Urgent Warning Of Potential Power Outages
May 20, 2025
