Novavax COVID-19 Vaccine Gets FDA Nod, Facing Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
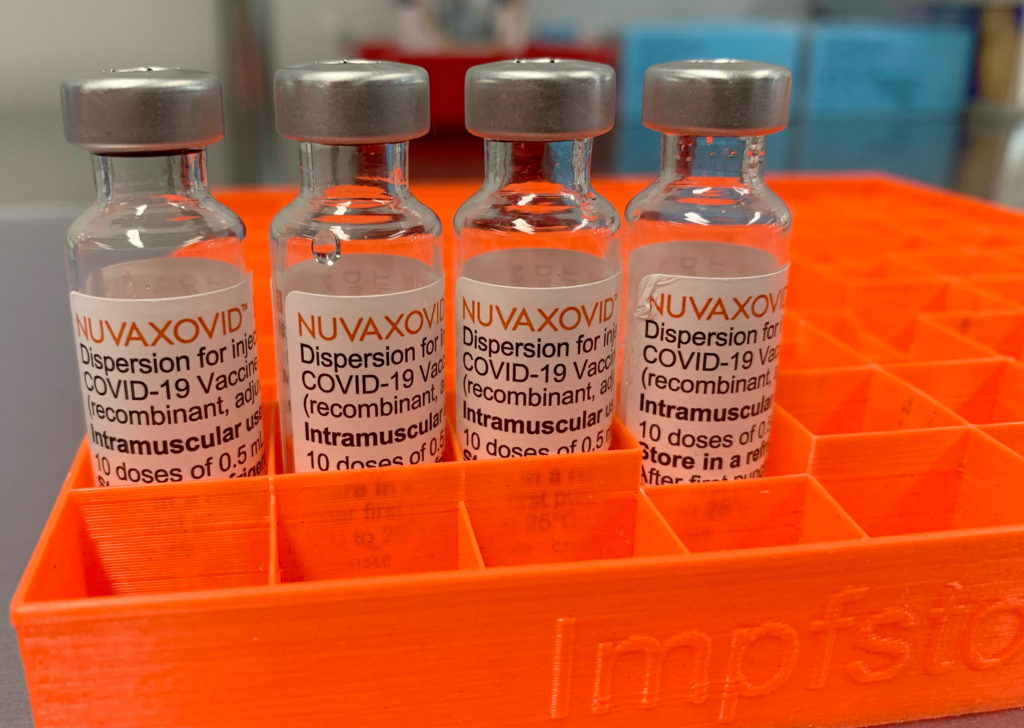
Novavax COVID-19 Vaccine Gets FDA Nod, but Faces Usage Restrictions
Novavax's COVID-19 vaccine, Nuvaxovid, finally receives FDA approval, but with significant limitations impacting its widespread use. After months of anticipation and delays, the FDA has granted full approval to Novavax's COVID-19 vaccine for individuals 18 years and older. However, this approval comes with caveats that significantly restrict its potential market share and raise questions about its future role in the ongoing pandemic response.
The approval marks a significant milestone for Novavax, which has faced numerous setbacks in bringing its protein-subunit vaccine to market. Unlike mRNA vaccines like those from Pfizer-BioNTech and Moderna, Nuvaxovid utilizes a more traditional technology, employing a purified protein antigen to trigger an immune response. This approach has been touted by some as potentially less prone to adverse effects, although real-world data is still emerging.
Restricted Usage: A Key Limitation
The FDA's approval is not without its limitations. The agency's decision comes with specific usage restrictions, primarily due to the limited demand for a new COVID-19 vaccine in the current landscape. With widespread availability of mRNA vaccines and substantial population immunity, the need for an additional vaccine is less urgent. This contrasts sharply with the initial high demand seen during the early stages of the pandemic.
Key restrictions include:
- Limited demand: The FDA acknowledges the decreased demand for COVID-19 vaccines, impacting the overall usage of Nuvaxovid.
- Competition with existing vaccines: The existing mRNA vaccines and other authorized vaccines already occupy a significant portion of the market.
- Potential for vaccine hesitancy: Some individuals may still exhibit hesitancy towards receiving any COVID-19 vaccine, potentially further limiting Nuvaxovid's uptake.
- Specific age group restriction: The approval is currently limited to individuals aged 18 and older. Further trials and data are needed to determine its safety and efficacy in younger populations.
Novavax's Future in the COVID-19 Landscape
Despite the restrictions, Novavax remains optimistic about the vaccine's future. The company points to the potential for its vaccine to serve as a valuable tool in specific populations, particularly those who may have concerns about mRNA vaccines or who prefer a more traditional vaccine technology. Further research and clinical trials are planned to explore the vaccine's potential use in younger age groups and as a booster shot.
This approval also opens the door for global distribution, potentially addressing vaccine inequities in lower-income countries where access to mRNA vaccines remains limited. The World Health Organization (WHO) has already pre-qualified Nuvaxovid, facilitating its wider deployment globally.
Looking Ahead: The Role of Protein-Subunit Vaccines
The FDA approval of Nuvaxovid provides a renewed focus on protein-subunit vaccine technology. While mRNA vaccines have dominated the COVID-19 vaccination landscape, protein-subunit vaccines offer a different approach, potentially addressing concerns about mRNA technology's novel nature. Further research and development in this area could lead to the creation of more effective and accessible vaccines for future pandemics.
The FDA's decision underscores the complexities of navigating the evolving COVID-19 pandemic response. While the approval of Nuvaxovid offers another option, the inherent limitations highlight the need for ongoing monitoring and adaptation in vaccine strategies. The long-term impact of Nuvaxovid on global vaccination efforts remains to be seen. However, its approval marks a significant step forward in the ongoing battle against COVID-19.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, protein-subunit vaccine, mRNA vaccine, vaccine restrictions, vaccine hesitancy, global vaccination, pandemic response, WHO pre-qualification.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, Facing Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Federal Reserves 2025 Rate Cut Outlook Implications For Us Treasury Yields
May 20, 2025
Federal Reserves 2025 Rate Cut Outlook Implications For Us Treasury Yields
May 20, 2025 -
 Experience The Intensity Daniel Craig Cillian Murphy And Tom Hardy In A Must See Wwi Drama
May 20, 2025
Experience The Intensity Daniel Craig Cillian Murphy And Tom Hardy In A Must See Wwi Drama
May 20, 2025 -
 Jenn Sterger Revisits The Brett Favre Scandal A Story Of Exploitation And Neglect
May 20, 2025
Jenn Sterger Revisits The Brett Favre Scandal A Story Of Exploitation And Neglect
May 20, 2025 -
 Thousands Of Airbnb Rentals In Spain Shut Down Due To Regulatory Issues
May 20, 2025
Thousands Of Airbnb Rentals In Spain Shut Down Due To Regulatory Issues
May 20, 2025 -
 Repaso A La Semana De Carreras Del Real Madrid Resultados Y Desempeno
May 20, 2025
Repaso A La Semana De Carreras Del Real Madrid Resultados Y Desempeno
May 20, 2025
