Novavax COVID-19 Vaccine Gets FDA Nod, Facing Unusual Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Gets FDA Nod, Facing Unusual Usage Restrictions
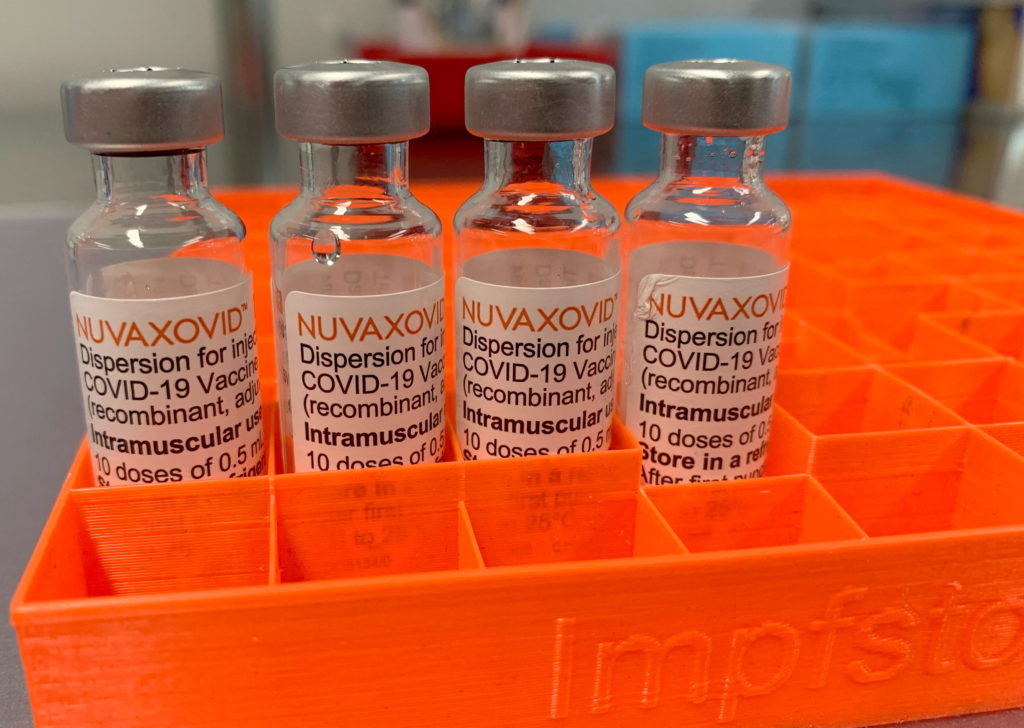
The FDA has finally approved the Novavax COVID-19 vaccine, but with a significant caveat: its use is severely restricted. This long-awaited approval comes with unusual limitations, raising questions about its market impact and the future of COVID-19 vaccination strategies. While many hailed the news as a victory for vaccine diversity, the stringent conditions attached are prompting significant discussion within the medical community.
The approval, announced on July 13, 2023, marks a significant milestone for Novavax, whose protein-subunit vaccine represents a different technological approach compared to the mRNA vaccines from Pfizer-BioNTech and Moderna. This difference was touted as a potential boon for vaccine hesitancy, offering an alternative for individuals wary of mRNA technology. However, the FDA's decision to approve it only for adults aged 18 years and older and with specific usage restrictions, casts a shadow on its widespread adoption.
Why the Restricted Approval?
The FDA's decision is rooted in several factors. While the vaccine demonstrated efficacy and safety in clinical trials, its performance is arguably less impressive than that of its mRNA counterparts. Additionally, the production process, while unique, faced challenges and delays, impacting the vaccine's timely rollout. The limited data available on the vaccine's effectiveness against newer variants, such as Omicron subvariants, also played a role in the FDA’s cautious approach.
The agency's approval emphasizes a "limited authorization" approach, meaning the vaccine's use will primarily focus on specific populations. This contrasts sharply with the broader authorizations given to mRNA vaccines. The agency recommends using the vaccine only when the individual cannot receive, or refuses, other authorized or recommended COVID-19 vaccines.
What this Means for Public Health
The limited authorization poses a significant challenge to broader vaccination efforts. While offering a technological alternative, the Novavax vaccine's restricted use may not substantially shift vaccination rates. The complexities surrounding its rollout and the specific criteria for administration could limit its accessibility.
Experts suggest that the limited approval underscores the challenges inherent in developing and deploying vaccines, even in the face of a global pandemic. The FDA's decision highlights the rigorous standards required for vaccine approval and the ongoing need for robust clinical data to support widespread use.
The Future of Novavax and COVID-19 Vaccination
The future of the Novavax vaccine remains uncertain. Its impact on overall vaccination rates might be minimal given the existing availability of other, more widely accessible, and arguably more effective vaccines. However, its approval does offer a backup option for those who specifically request or require a non-mRNA vaccine. The vaccine's efficacy against future variants will also be crucial in determining its long-term viability.
Further research and monitoring of the vaccine's real-world performance are necessary to fully assess its impact on public health. This includes ongoing surveillance for side effects and a continuous evaluation of its effectiveness against emerging variants. The FDA's decision, although cautiously optimistic, underscores the complexities of vaccine development and deployment in a dynamic pandemic environment.
Keywords: Novavax, COVID-19 vaccine, FDA approval, vaccine restrictions, mRNA vaccine, protein-subunit vaccine, vaccine hesitancy, public health, COVID-19 vaccination, Omicron variant, vaccine efficacy, limited authorization.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, Facing Unusual Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Investing In The Future The Importance Of Federal Funding For Medical And Scientific Research In America
May 21, 2025
Investing In The Future The Importance Of Federal Funding For Medical And Scientific Research In America
May 21, 2025 -
 Behind The Bestsellers The Taylor Jenkins Reid Phenomenon
May 21, 2025
Behind The Bestsellers The Taylor Jenkins Reid Phenomenon
May 21, 2025 -
 A Candid Look At Jamie Lee Curtis Relationship With Lindsay Lohan
May 21, 2025
A Candid Look At Jamie Lee Curtis Relationship With Lindsay Lohan
May 21, 2025 -
 Charlotte Residents Urged To Prepare For Tonights Severe Storms And Following Cold Snap
May 21, 2025
Charlotte Residents Urged To Prepare For Tonights Severe Storms And Following Cold Snap
May 21, 2025 -
 Watch Now A Powerful Wwi Film Starring Daniel Craig Cillian Murphy And Tom Hardy
May 21, 2025
Watch Now A Powerful Wwi Film Starring Daniel Craig Cillian Murphy And Tom Hardy
May 21, 2025
