Novavax COVID-19 Vaccine Gets FDA Nod, But With Uncommon Conditions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Gets FDA Nod, but with Uncommon Conditions
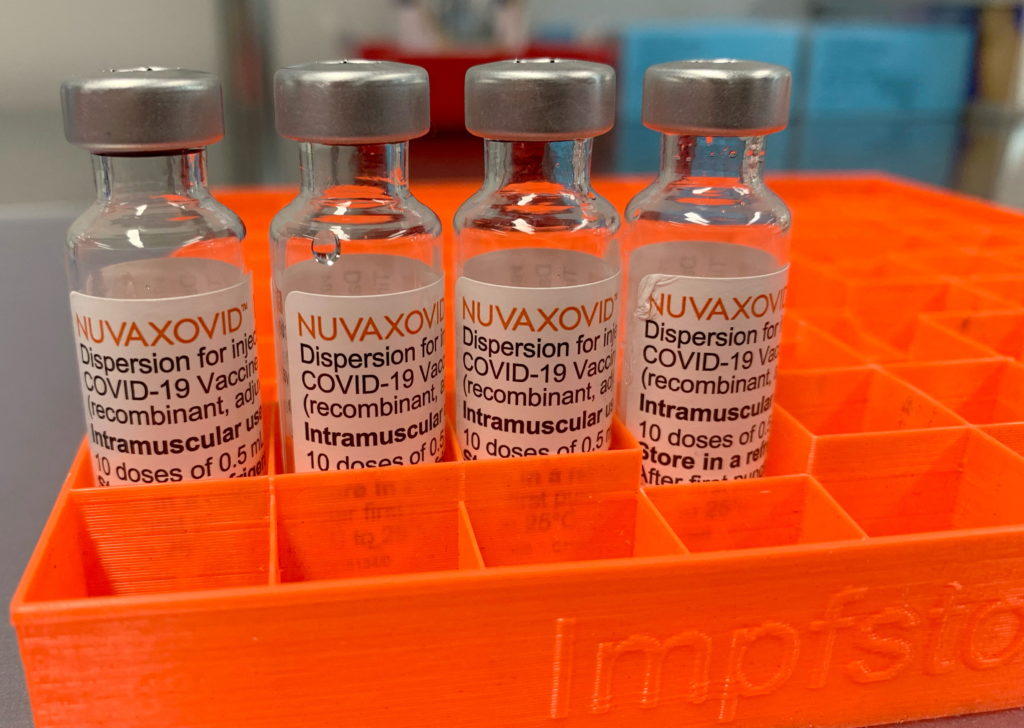
The FDA has finally granted full approval to the Novavax COVID-19 vaccine, Nuvaxovid, marking a significant step in the fight against the pandemic. However, this approval comes with some caveats, raising questions about its place in the current vaccination landscape. The approval, while welcomed by some, is tempered by the inclusion of uncommon but serious side effects detailed in the FDA's assessment.
A Long-Awaited Approval, but with Conditions
The Novavax vaccine, a protein subunit vaccine different from the mRNA vaccines like Pfizer-BioNTech and Moderna, has been authorized for emergency use in several countries for some time. Its arrival in the US market has been eagerly anticipated by a segment of the population hesitant about mRNA technology. The FDA's full approval provides a significant boost of confidence for those individuals. However, the agency's announcement also highlights a crucial detail: the vaccine's approval comes with a detailed listing of potential side effects, including some considered uncommon but serious.
Understanding the Side Effects Profile
The FDA's review process uncovered instances of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining around the heart) following vaccination with Nuvaxovid. While these events are considered uncommon, their occurrence necessitates careful monitoring and informed consent. The agency has stressed the importance of healthcare professionals being aware of these potential side effects and advising patients accordingly. Further research into the exact incidence rate of these conditions is ongoing.
How Does Novavax Differ from Other COVID-19 Vaccines?
Unlike the mRNA vaccines that use messenger RNA to instruct cells to produce a viral protein, the Novavax vaccine uses a different approach. It utilizes a protein subunit technology, employing a lab-created version of the spike protein found on the surface of the SARS-CoV-2 virus. This protein is then combined with an adjuvant to enhance the immune response. This difference in technology may appeal to individuals who have expressed concerns about the mRNA vaccines, although the effectiveness of all authorized vaccines remains paramount.
The Future of Novavax in the COVID-19 Vaccination Strategy
The FDA's approval, albeit conditional, solidifies Novavax's role in the broader COVID-19 vaccination strategy. The availability of a protein subunit vaccine expands options for individuals who may have previously been hesitant due to concerns about mRNA technology. However, the acknowledged uncommon but serious side effects necessitate a careful consideration of the risk-benefit profile for each individual. Healthcare providers will play a vital role in ensuring patients are fully informed before receiving the vaccine.
Further Research and Ongoing Monitoring
The FDA's approval is not the end of the story. Ongoing monitoring of the vaccine's safety and efficacy is crucial. The agency will continue to analyze data from post-market surveillance to identify any further safety concerns and refine the understanding of its risk-benefit profile. This commitment to ongoing monitoring underscores the importance of a data-driven approach to vaccine safety. This commitment to ongoing research and monitoring ensures that the public receives the most up-to-date and accurate information regarding the Novavax vaccine.
Conclusion:
The FDA's full approval of the Novavax COVID-19 vaccine represents a significant development. However, the acknowledgement of uncommon but serious side effects underscores the importance of informed decision-making. Individuals should consult with their healthcare providers to discuss the potential risks and benefits of the Novavax vaccine in the context of their individual health circumstances and pre-existing conditions. The vaccine's availability offers a wider choice, but a well-informed choice remains key to successful public health initiatives.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, protein subunit vaccine, mRNA vaccine, side effects, myocarditis, pericarditis, vaccine safety, vaccination, public health, COVID-19 pandemic.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, But With Uncommon Conditions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 What A Gleason Score Of 9 Means For Prostate Cancer Patients
May 20, 2025
What A Gleason Score Of 9 Means For Prostate Cancer Patients
May 20, 2025 -
 Jamie Lee Curtis Defends Lindsay Lohan A Longstanding Friendship
May 20, 2025
Jamie Lee Curtis Defends Lindsay Lohan A Longstanding Friendship
May 20, 2025 -
 Putins Actions Underscore Trumps Waning International Power
May 20, 2025
Putins Actions Underscore Trumps Waning International Power
May 20, 2025 -
 Teaser Talk Unraveling The Story Behind Ntrs Global Travels
May 20, 2025
Teaser Talk Unraveling The Story Behind Ntrs Global Travels
May 20, 2025 -
 Stock Market Rally Continues Six Day Win Streak For S And P 500 Amidst Moodys Action
May 20, 2025
Stock Market Rally Continues Six Day Win Streak For S And P 500 Amidst Moodys Action
May 20, 2025
