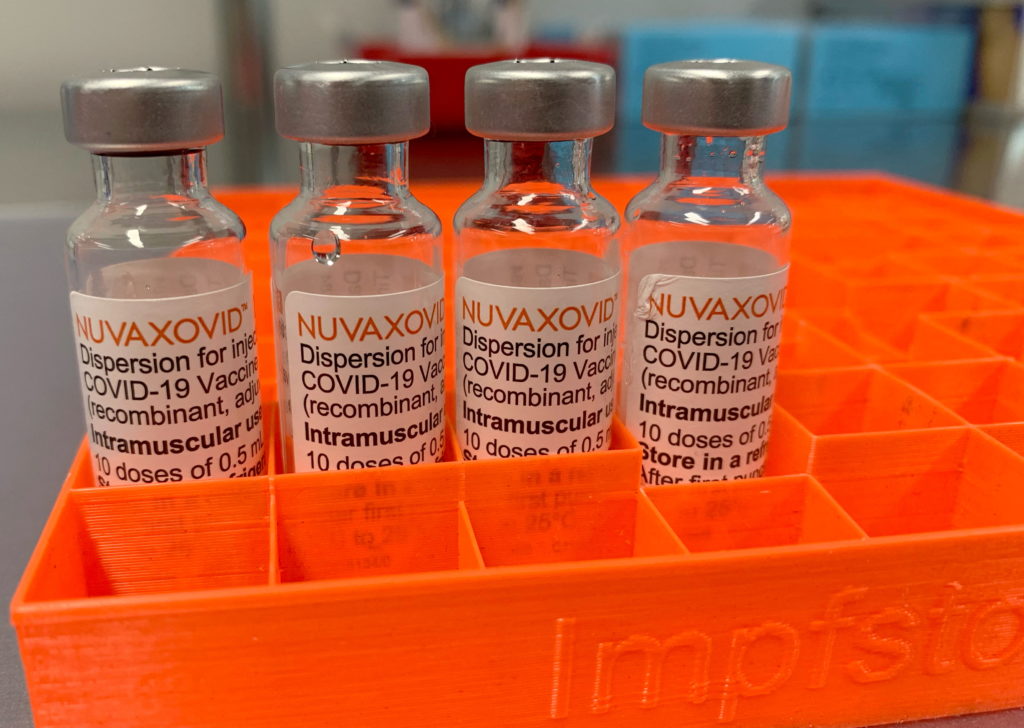
Novavax COVID-19 Vaccine Gets FDA Nod, But With Significant Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Gets FDA Nod, But With Significant Usage Restrictions
Novavax's COVID-19 vaccine, Nuvaxovid, has finally received Emergency Use Authorization (EUA) from the FDA, but its rollout will be significantly hampered by usage restrictions. This long-awaited approval comes with caveats that limit its potential impact on the ongoing pandemic response. While many hail the news as a welcome addition to the vaccine arsenal, the limitations raise questions about its practical application.
The FDA's decision, announced [Insert Date], grants EUA for Nuvaxovid for individuals 18 years of age and older. However, the agency's statement clearly outlined specific limitations, significantly impacting its potential widespread use.
Key Restrictions Limiting Nuvaxovid's Impact
-
Limited Target Population: Unlike other widely used COVID-19 vaccines, Nuvaxovid's authorization is currently only for adults. This excludes a significant portion of the population, particularly children and adolescents, who remain vulnerable to infection. Further studies are needed to determine its safety and efficacy in younger age groups.
-
Conditional Authorization: The EUA is conditional upon Novavax meeting ongoing requirements, including continued monitoring of safety data and post-market surveillance. This highlights the ongoing uncertainty surrounding the long-term effects of the vaccine. Failure to meet these conditions could lead to revocation of the EUA.
-
Lower Efficacy Compared to mRNA Vaccines: While Nuvaxovid has demonstrated efficacy against COVID-19, clinical trial data suggests its effectiveness might be lower compared to the mRNA vaccines (Pfizer-BioNTech and Moderna) currently dominating the market. This lower efficacy could affect its uptake among individuals already vaccinated or those considering their options. [Link to relevant clinical trial data].
-
Potential for Competition and Limited Demand: The landscape of COVID-19 vaccines has shifted significantly since Novavax initially sought approval. With high vaccination rates in many countries and the availability of readily accessible mRNA vaccines and boosters, the demand for a new vaccine with limitations may be limited.
Why the Approval Matters Despite Restrictions
Despite the significant usage restrictions, the FDA's approval of Nuvaxovid holds some importance. It provides an alternative for individuals who may have concerns about mRNA vaccines or have experienced adverse reactions to other available vaccines. This offers a valuable option for those hesitant to receive the currently available vaccines, potentially increasing overall vaccination rates. The protein-based technology used in Nuvaxovid differs from the mRNA technology used in other vaccines, and its approval diversifies the available vaccine portfolio.
Future Prospects and Ongoing Challenges
Novavax faces the challenge of navigating a market already saturated with effective COVID-19 vaccines. The company will need a robust marketing strategy to communicate the vaccine's benefits and address concerns about its limitations. Furthermore, securing widespread distribution and addressing potential logistical hurdles will be crucial for successful implementation. Future clinical trials targeting younger age groups could significantly broaden the vaccine's potential reach.
The FDA's decision represents a complex situation. While the addition of Nuvaxovid to the vaccine arsenal offers a potentially beneficial alternative for some, its restricted usage significantly limits its overall impact on the global COVID-19 pandemic response. The long-term effects and market acceptance of Nuvaxovid remain to be seen. Only time will tell if this newly approved vaccine will become a significant player in the fight against COVID-19.
Keywords: Novavax, COVID-19 vaccine, Nuvaxovid, FDA approval, Emergency Use Authorization, EUA, vaccine restrictions, protein-based vaccine, mRNA vaccine, vaccine efficacy, vaccine safety, COVID-19 pandemic, public health.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Gets FDA Nod, But With Significant Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Beyond The Game Exploring The Evolving Joel And Ellie Relationship In The Last Of Us Season 2
May 21, 2025
Beyond The Game Exploring The Evolving Joel And Ellie Relationship In The Last Of Us Season 2
May 21, 2025 -
 Messages Of Hope For President Biden After Cancer Diagnosis Announcement
May 21, 2025
Messages Of Hope For President Biden After Cancer Diagnosis Announcement
May 21, 2025 -
 Fbi Director Confirms Investigation Into New York Attorney Generals Office
May 21, 2025
Fbi Director Confirms Investigation Into New York Attorney Generals Office
May 21, 2025 -
 League Of Legends Hall Of Fame 2025 Player Reaction To The Latest Induction
May 21, 2025
League Of Legends Hall Of Fame 2025 Player Reaction To The Latest Induction
May 21, 2025 -
 League Of Legends Hall Of Fame 2025 Concerns Over Potential Skin Price
May 21, 2025
League Of Legends Hall Of Fame 2025 Concerns Over Potential Skin Price
May 21, 2025
