Novavax COVID-19 Vaccine: FDA Grants Approval Under Specific Circumstances
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine: FDA Grants Approval Under Specific Circumstances
The FDA's conditional approval of the Novavax COVID-19 vaccine offers a new option for some, but with limitations.
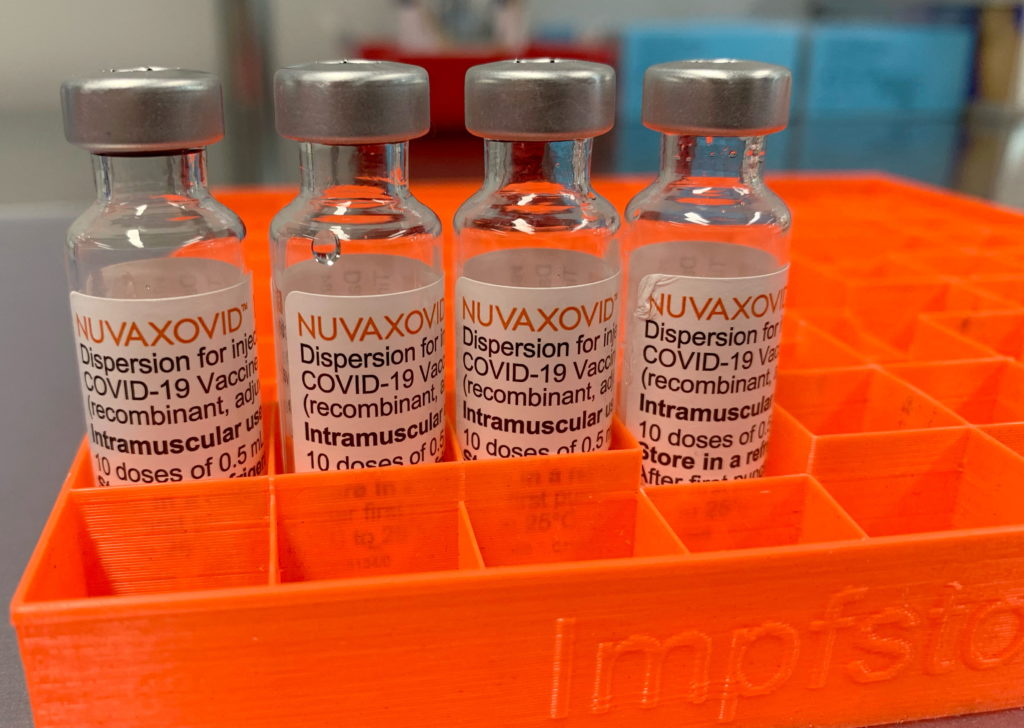
The US Food and Drug Administration (FDA) has granted Emergency Use Authorization (EUA) to the Novavax COVID-19 vaccine, Nuvaxovid, for individuals 18 years of age and older. This announcement, while welcomed by some, comes with crucial caveats regarding its specific applications and the existing landscape of COVID-19 vaccination. The approval isn't a blanket endorsement, but rather a targeted one, addressing a gap in the market for a protein-based vaccine option.
This development marks a significant shift in the COVID-19 vaccine landscape, providing an alternative to the mRNA vaccines (like Pfizer-BioNTech and Moderna) and the viral vector vaccine (Johnson & Johnson). The Novavax vaccine uses a different technology – a protein subunit approach – which some individuals may find preferable due to its more traditional methodology. This protein-based technology could be particularly appealing to those hesitant about mRNA vaccines.
Understanding the Nuvaxovid Approval: Why the Specific Circumstances?
The FDA's decision wasn't based on a complete disregard for existing vaccines. Instead, the approval hinges on several factors:
- Addressing Vaccine Hesitancy: A significant portion of the population remains hesitant about receiving a COVID-19 vaccine. The availability of a different vaccine type, like Nuvaxovid, might encourage these individuals to get vaccinated, ultimately contributing to broader community immunity.
- Specific Population Needs: While the EUA covers adults 18 and older, further research and potential future approvals might extend its use to other age groups. The FDA is currently evaluating its efficacy and safety in other demographics.
- Boosting Vaccine Supply: Adding another vaccine to the market increases overall supply, which could be particularly beneficial during periods of high demand or vaccine shortages.
How Nuvaxovid Works: A Protein Subunit Approach
Unlike mRNA vaccines, which introduce mRNA to instruct cells to produce a viral protein, the Novavax vaccine uses purified fragments of the SARS-CoV-2 virus’s spike protein. This protein is produced using a baculovirus expression system in insect cells. The body then mounts an immune response to this protein, generating antibodies that protect against infection. This traditional method may appeal to those concerned about the newer mRNA technology.
What are the potential side effects?
Common side effects reported during clinical trials included pain, tenderness, or swelling at the injection site, fatigue, muscle aches, and headaches. Serious side effects were rare. As with all vaccines, potential risks and benefits should be discussed with a healthcare provider.
Where can I get the Novavax vaccine?
The availability of the Novavax vaccine will vary by location. It's recommended to check with your doctor or local health department to determine availability in your area. You can also find updated information on the .
Looking Ahead: The Future of Novavax and COVID-19 Vaccination
The FDA's conditional approval of the Novavax vaccine represents a significant step forward in the fight against COVID-19. While it's not a panacea, its availability provides a valuable option for those hesitant about other vaccines, potentially increasing overall vaccination rates and contributing to stronger community protection. Further research and monitoring of its long-term efficacy and safety are ongoing. Stay informed and consult your healthcare provider for personalized advice on COVID-19 vaccination.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, protein subunit vaccine, mRNA vaccine, vaccine hesitancy, COVID-19 vaccination, vaccine safety, vaccine efficacy, coronavirus vaccine, COVID-19 pandemic.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Grants Approval Under Specific Circumstances. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Exclusive Interview Jamie Lee Curtis On Maintaining Her Bond With Lindsay Lohan
May 20, 2025
Exclusive Interview Jamie Lee Curtis On Maintaining Her Bond With Lindsay Lohan
May 20, 2025 -
 Helldivers 2 Masters Of Ceremony Warbond Drop Details For May 15th
May 20, 2025
Helldivers 2 Masters Of Ceremony Warbond Drop Details For May 15th
May 20, 2025 -
 American Leadership How Medical And Scientific Research Drives National Greatness
May 20, 2025
American Leadership How Medical And Scientific Research Drives National Greatness
May 20, 2025 -
 A J Perez On The Pressure And Threats Surrounding The Brett Favre Untold Documentary
May 20, 2025
A J Perez On The Pressure And Threats Surrounding The Brett Favre Untold Documentary
May 20, 2025 -
 Live Score And Drama Lsg Vs Srh Ipl 2025 Match Highlights And Controversy
May 20, 2025
Live Score And Drama Lsg Vs Srh Ipl 2025 Match Highlights And Controversy
May 20, 2025
