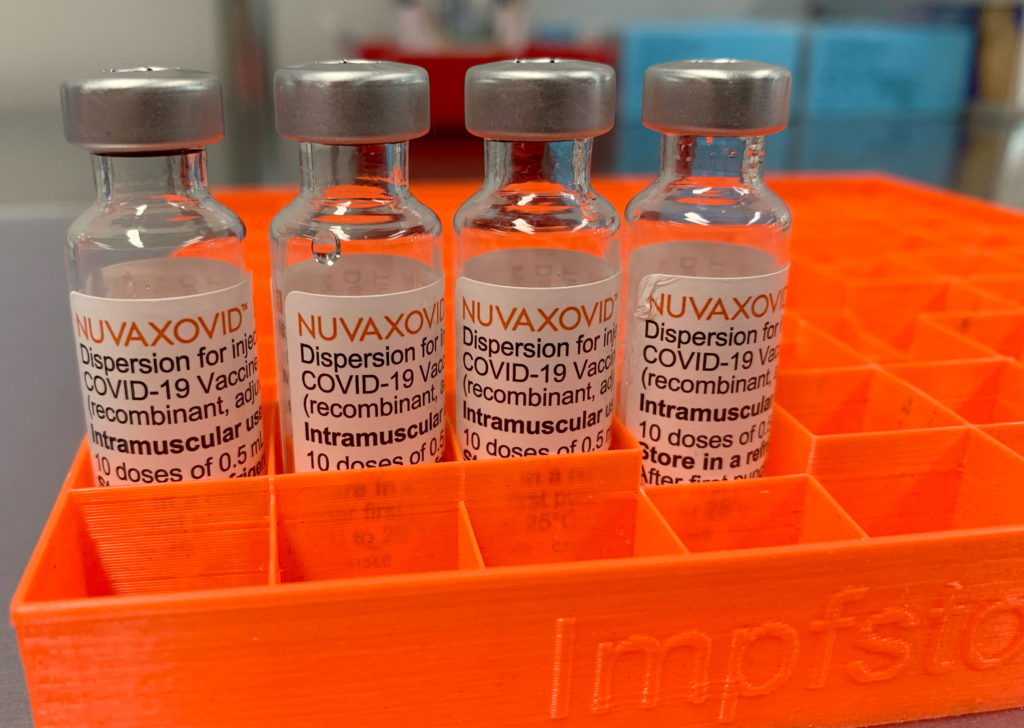
Novavax COVID-19 Vaccine: FDA Approval Includes Unusual Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine: FDA Approval Includes Unusual Usage Restrictions
The Novavax COVID-19 vaccine, Nuvaxovid, finally received FDA approval in July 2023, marking a significant milestone for the company. However, this approval comes with a caveat: unusual and arguably restrictive usage guidelines that have sparked debate among health experts and the public. This article delves into the specifics of these restrictions and their potential impact on vaccine rollout and public health strategies.
What Makes Novavax's FDA Approval Different?
Unlike the previously authorized mRNA vaccines (Pfizer-BioNTech and Moderna) and the Johnson & Johnson viral vector vaccine, the FDA's approval of Novavax includes a significant limitation: it's primarily intended for individuals who cannot or will not receive other authorized or approved COVID-19 vaccines. This "last resort" designation is unprecedented and raises concerns about its practical implications.
Understanding the Usage Restrictions:
The FDA's decision is based on several factors. While Novavax offers a different mechanism of action – using a protein-based approach instead of mRNA or viral vectors – its efficacy data, while showing effectiveness, doesn't surpass that of the already authorized vaccines. Furthermore, the available data suggests a lower efficacy against certain variants. The agency explicitly states that the vaccine should only be considered when other options aren't suitable.
This restriction could impact several groups:
- Individuals with specific pre-existing conditions: Those with severe allergies to mRNA or viral vector components might find Novavax a viable option, but even then, thorough consultation with a healthcare professional is crucial.
- Vaccine hesitancy: The protein-based approach might appeal to those hesitant about mRNA technology, though this is not a guarantee.
- Limited supply: The relatively limited manufacturing capacity of Novavax compared to other vaccine manufacturers could restrict its availability.
The Implications of Restricted Usage:
These restrictions raise several important questions:
- Accessibility: Will this limited usage restrict access for those who might benefit, even if they aren't in the explicitly defined "last resort" category?
- Public health strategy: How will this impact broader vaccination campaigns and the overall effort to achieve herd immunity?
- Vaccine hesitancy: Could this "last resort" designation further fuel vaccine hesitancy among those who might otherwise consider it?
- Resource allocation: Will limited resources be effectively allocated given the restricted usage guidelines?
Moving Forward: Challenges and Opportunities
The FDA's decision highlights the complexities of vaccine approval and distribution. While Novavax provides an alternative approach, the restrictive usage guidelines present significant challenges. Transparency and clear communication are crucial to ensure appropriate usage and avoid confusion.
Further research and data analysis are needed to better understand Novavax's long-term efficacy and safety profile. This information will be vital in guiding future public health strategies and determining the vaccine's ultimate role in the fight against COVID-19. The World Health Organization (WHO) continues to monitor the situation and provide guidance on global vaccine deployment. For updated information, please visit the and the .
Call to Action: Consult your healthcare provider to discuss which COVID-19 vaccine is best suited for your individual needs and circumstances. Staying informed about the latest vaccine information is crucial for making informed decisions about your health.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Approval Includes Unusual Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Tensions Rise Lsg And Srh Players Verbal Confrontation During Ipl 2025 Live Score
May 20, 2025
Tensions Rise Lsg And Srh Players Verbal Confrontation During Ipl 2025 Live Score
May 20, 2025 -
 Tuesday Briefing Assessing Israels Basic Food Aid Package
May 20, 2025
Tuesday Briefing Assessing Israels Basic Food Aid Package
May 20, 2025 -
 Trumps Dependence On Putin A Shifting Global Dynamic
May 20, 2025
Trumps Dependence On Putin A Shifting Global Dynamic
May 20, 2025 -
 Clean Energy Investment And Tax Incentives Fueling Economic Growth Or Inflation
May 20, 2025
Clean Energy Investment And Tax Incentives Fueling Economic Growth Or Inflation
May 20, 2025 -
 Ufc Fans React To Jon Jones I M Done Statement Aspinall Fight In Jeopardy
May 20, 2025
Ufc Fans React To Jon Jones I M Done Statement Aspinall Fight In Jeopardy
May 20, 2025
