Novavax COVID-19 Vaccine: FDA Approval Comes With Unexpected Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
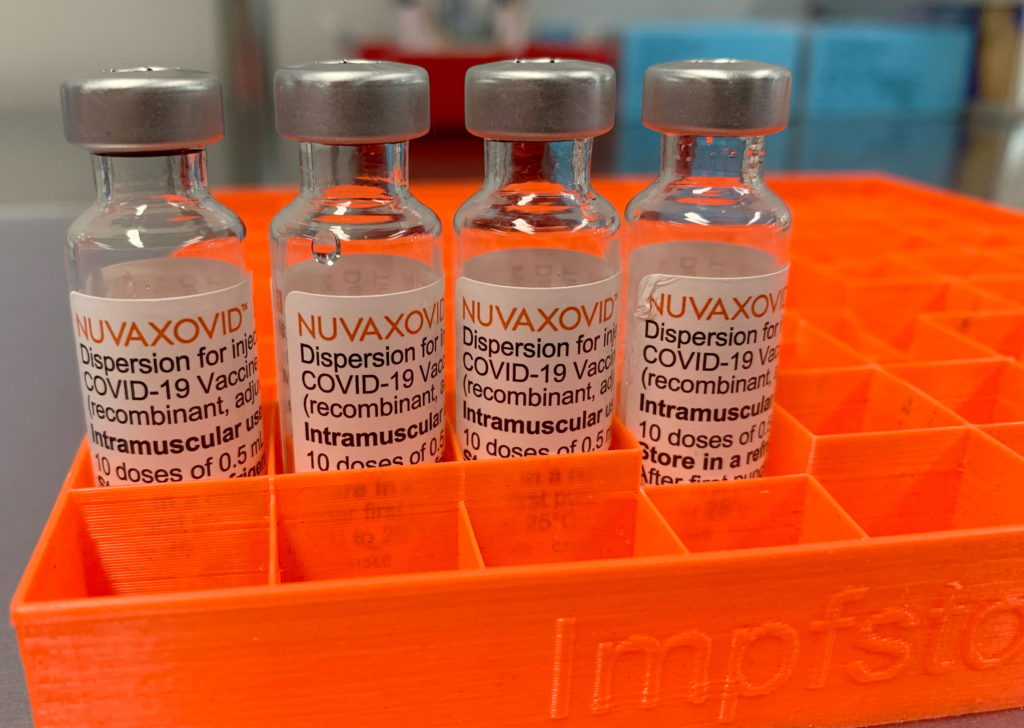
Novavax COVID-19 Vaccine: FDA Approval Comes with Unexpected Restrictions
The Novavax COVID-19 vaccine, Nuvaxovid, finally received FDA approval in July 2023, marking a significant milestone in the fight against the pandemic. However, this approval wasn't without its caveats. The FDA's decision came with unexpected and somewhat controversial restrictions, raising questions about the vaccine's future role in the US vaccination landscape. This article delves into the details of the approval, highlighting the limitations and their potential impact.
Limited Authorization, Not Full Approval:
It's crucial to understand that the FDA granted authorization, not full approval, for Nuvaxovid. This distinction is significant. While authorization allows for emergency use, full approval follows a more rigorous review process and typically indicates a higher level of confidence in the vaccine's safety and efficacy. The FDA's decision to grant authorization rather than full approval suggests lingering concerns that require further investigation.
Age Restrictions and Safety Concerns:
The FDA's authorization currently limits the use of the Novavax vaccine to individuals aged 18 years and older. This contrasts with other authorized COVID-19 vaccines, some of which are approved for use in younger age groups. While Novavax has presented data suggesting efficacy and safety across different age demographics, the FDA's decision suggests the need for more conclusive evidence before expanding its use to adolescents and children. Further studies are underway to address this limitation.
Supply Chain Issues and Limited Demand:
Beyond regulatory hurdles, the Novavax vaccine has faced significant challenges related to its production and distribution. Supply chain issues have impacted the vaccine's availability, making it less accessible compared to other widely available COVID-19 vaccines. This limited supply, coupled with waning public interest in COVID-19 vaccination, presents a considerable obstacle to its widespread adoption.
The Role of Vaccine Hesitancy:
The hesitancy surrounding COVID-19 vaccines, in general, has played a significant role in the slower uptake of the Novavax vaccine. Some individuals have expressed preferences for specific vaccine types based on their composition or perceived side effects. The protein-based nature of the Novavax vaccine, while potentially appealing to some hesitant individuals, hasn't been sufficient to overcome the broader challenges of vaccine hesitancy. Public health officials are working to address these concerns through improved communication and education campaigns.
Looking Ahead: The Future of Nuvaxovid in the US:
The FDA's conditional approval of the Novavax COVID-19 vaccine, while a step forward, comes with significant restrictions. The limited age range, ongoing concerns regarding efficacy and safety data, and the existing challenges associated with supply and vaccine hesitancy all contribute to an uncertain future for Nuvaxovid in the United States. Further research and data collection will be critical in determining the long-term role of this vaccine in the broader COVID-19 vaccination strategy. Continued monitoring of its safety and efficacy profile will be essential for informing future decisions about its use.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, vaccine restrictions, vaccine hesitancy, protein-based vaccine, supply chain issues, COVID-19 vaccination, vaccine safety, vaccine efficacy.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Approval Comes With Unexpected Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Walmart Warns Of Price Increases After Trumps Tariff Comments
May 20, 2025
Walmart Warns Of Price Increases After Trumps Tariff Comments
May 20, 2025 -
 Peaky Blinders Returns Confirmed New Series And A Big Twist
May 20, 2025
Peaky Blinders Returns Confirmed New Series And A Big Twist
May 20, 2025 -
 Top Mlb Predictions Best Bets And Picks For Monday May 19 2025
May 20, 2025
Top Mlb Predictions Best Bets And Picks For Monday May 19 2025
May 20, 2025 -
 Tom Aspinall Responds Analyzing The Backlash To Jon Jones Strip The Duck Statement
May 20, 2025
Tom Aspinall Responds Analyzing The Backlash To Jon Jones Strip The Duck Statement
May 20, 2025 -
 Jamie Lee Curtis On Lindsay Lohan A Longstanding Friendship Revealed
May 20, 2025
Jamie Lee Curtis On Lindsay Lohan A Longstanding Friendship Revealed
May 20, 2025
