Novavax COVID-19 Vaccine: FDA Approval Comes With Strict Usage Guidelines
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine: FDA Approval Comes with Strict Usage Guidelines
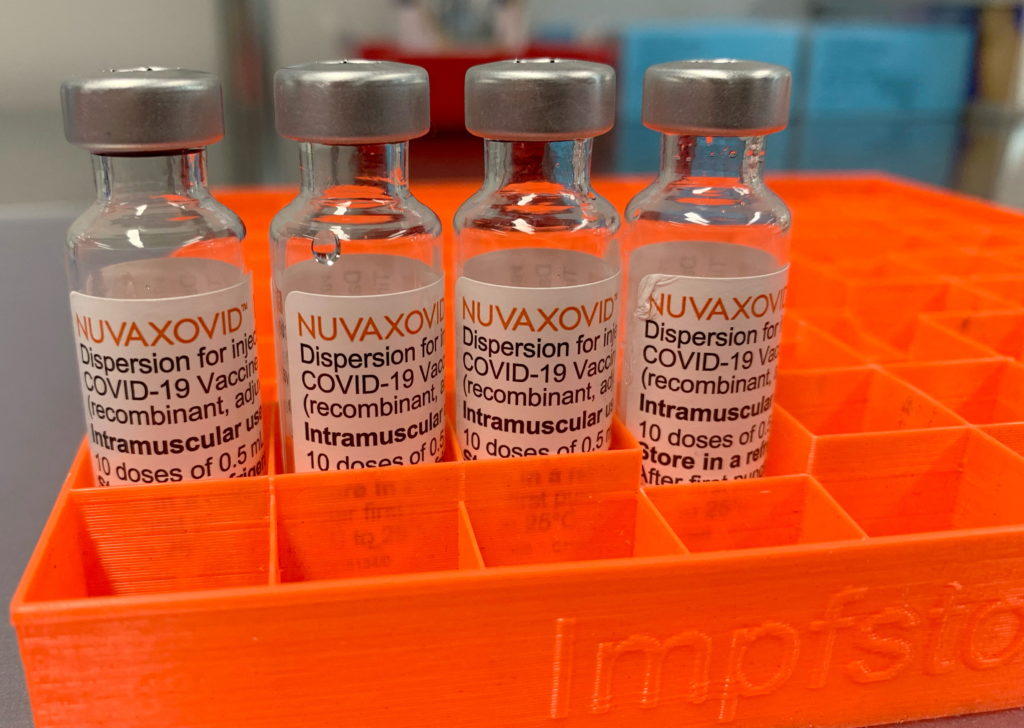
The Novavax COVID-19 vaccine, Nuvaxovid, has finally received FDA approval, marking a significant milestone in the fight against the pandemic. However, this approval isn't without caveats. The FDA's authorization comes with stringent usage guidelines, limiting its availability and raising questions about its overall impact. This article delves into the details of the approval, the specific guidelines imposed, and what this means for the future of COVID-19 vaccination strategies.
A Closer Look at the FDA Approval
After months of review, the FDA granted full approval to Nuvaxovid, a protein-subunit vaccine. This differs significantly from the mRNA vaccines developed by Pfizer-BioNTech and Moderna. Novavax uses a more traditional approach, employing antigens derived from the virus's spike protein to trigger an immune response. This technology has been used for decades in other vaccines, potentially appealing to vaccine-hesitant individuals wary of mRNA technology. The approval, however, is not blanket authorization for all age groups.
Strict Usage Guidelines: Who Can Get the Novavax Vaccine?
While full FDA approval signifies a high degree of safety and efficacy, the agency has imposed specific limitations on Nuvaxovid's use. Currently, the vaccine is only authorized for individuals 18 years of age and older. This contrasts with the mRNA vaccines, which are authorized for use in younger age groups. The FDA's decision reflects a cautious approach, prioritizing safety data and focusing on the adult population initially. Further studies are likely needed to assess the vaccine's efficacy and safety in younger age groups.
The FDA's decision to approve only for adults aged 18 and above also affects its use as a booster. While the initial two-dose regimen is authorized, the FDA hasn't yet approved it as a booster shot for any age group. This means it's not currently an option for individuals seeking additional protection beyond their initial vaccination series.
Why the Strict Guidelines? Addressing Safety Concerns
The strict usage guidelines stem from the FDA's rigorous review process. While the vaccine demonstrated efficacy in clinical trials, the data may not be as extensive as that for the mRNA vaccines. The FDA's cautious approach prioritizes comprehensive safety data before broadening authorization. This highlights the stringent standards applied to all vaccines approved for use in the United States. The agency continually monitors the safety and effectiveness of all authorized vaccines, and guidelines may be adjusted as more data becomes available.
The Future of Novavax and COVID-19 Vaccination
The FDA's approval of the Novavax vaccine, despite the limitations, is a positive step. The availability of a protein-subunit vaccine offers an alternative for those who prefer this technology or have concerns about mRNA vaccines. However, the strict usage guidelines significantly impact its reach and immediate impact on overall vaccination rates. Ongoing clinical trials and further data analysis will be crucial in determining the future role of Nuvaxovid in the broader COVID-19 vaccination strategy, including potential authorization for booster shots and younger age groups. The FDA's commitment to continuous monitoring ensures that the safety and efficacy of the vaccine remain under close scrutiny.
Further Reading:
Call to Action: Consult your healthcare provider to determine the best COVID-19 vaccination strategy for you. Stay informed about the latest updates on vaccine approvals and guidelines from reliable sources like the FDA and CDC.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Approval Comes With Strict Usage Guidelines. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Confirmed A New Peaky Blinders Series Is Coming But With One Key Difference
May 21, 2025
Confirmed A New Peaky Blinders Series Is Coming But With One Key Difference
May 21, 2025 -
 Ufc News Jon Jones Latest Comments On Tom Aspinall Cause A Stir
May 21, 2025
Ufc News Jon Jones Latest Comments On Tom Aspinall Cause A Stir
May 21, 2025 -
 Pectra Upgrade Fuels Ethereum Investment 200 Million Rush
May 21, 2025
Pectra Upgrade Fuels Ethereum Investment 200 Million Rush
May 21, 2025 -
 Nature Conservation Drives Corporate Value Growth For 160 Japanese Companies New Sector Specific Guidelines Unveiled
May 21, 2025
Nature Conservation Drives Corporate Value Growth For 160 Japanese Companies New Sector Specific Guidelines Unveiled
May 21, 2025 -
 Juvenile Arrests Follow Church Break In And Bathroom Damage
May 21, 2025
Juvenile Arrests Follow Church Break In And Bathroom Damage
May 21, 2025
