Novavax COVID-19 Vaccine: FDA Approval Comes With Strict Conditions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
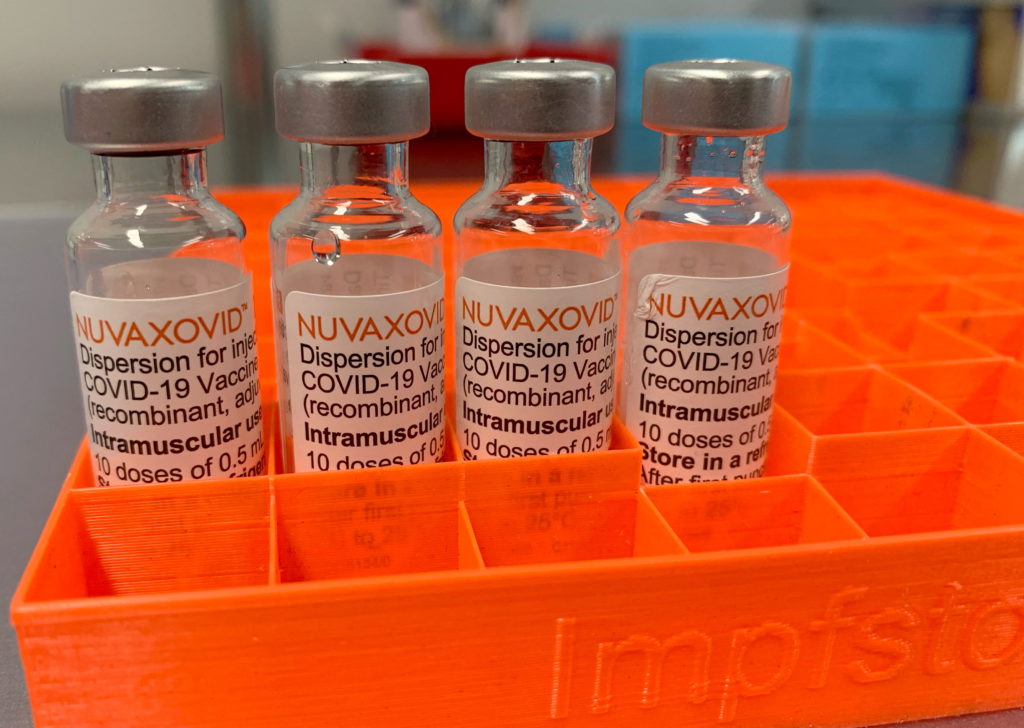
Novavax COVID-19 Vaccine: FDA Approval Comes with Strict Conditions
The Novavax COVID-19 vaccine, Nuvaxovid, finally received FDA approval in July 2023, but this landmark achievement comes with significant caveats. While celebrated by some as offering a different technological approach to COVID-19 prevention, the approval is accompanied by strict conditions that impact its rollout and overall efficacy perception. This article delves into the details of the FDA's approval, highlighting the conditions imposed and their implications for public health.
A Protein Subunit Vaccine: A Different Approach
Unlike mRNA vaccines like Pfizer-BioNTech and Moderna, or the viral vector vaccine from Johnson & Johnson, Nuvaxovid is a protein subunit vaccine. This technology uses a lab-made piece of the virus (the spike protein) to trigger an immune response. This approach has been used for years in other vaccines, potentially leading to increased public trust for some hesitant individuals. However, this familiarity doesn't negate the need for rigorous testing and safety monitoring.
FDA Approval: The Conditions
The FDA's approval wasn't a blanket endorsement. The agency imposed several strict conditions, significantly impacting the vaccine's immediate availability and broader implications:
-
Limited Authorization: The approval is specifically for individuals 18 years of age and older. Further testing and data are required before authorization can be expanded to younger age groups.
-
Stringent Manufacturing Oversight: The FDA will maintain rigorous oversight of the manufacturing process to ensure consistent quality and safety. This underscores concerns regarding potential production challenges and delays.
-
Post-Market Surveillance: Extensive post-market surveillance is mandated to continuously monitor the vaccine's safety and effectiveness. This ongoing monitoring is crucial, especially given the relatively late entry of Nuvaxovid into the market.
-
Data Requirements: Novavax is required to continue submitting data on the vaccine's long-term efficacy and safety profile. This demonstrates the FDA's cautious approach and commitment to ongoing assessment.
Implications for Public Health
The conditional approval of Nuvaxovid presents a complex picture for public health strategies. While offering an alternative vaccine technology, the strict conditions could hinder its widespread adoption. The limited authorization and stringent manufacturing oversight might restrict supply and increase logistical challenges. Furthermore, the ongoing need for data submission necessitates a continued evaluation of its role in overall COVID-19 vaccination efforts. This uncertainty could affect public confidence and vaccination uptake rates.
Looking Ahead:
The future of Nuvaxovid remains uncertain. Its success hinges on overcoming the challenges imposed by the conditional FDA approval. The effectiveness of post-market surveillance, the expansion of authorization to younger age groups, and overcoming potential manufacturing hurdles will all significantly influence the vaccine's ultimate impact on the global fight against COVID-19. Continuous monitoring of the situation and updates from health authorities are crucial for informed decision-making.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, protein subunit vaccine, vaccine safety, vaccine efficacy, post-market surveillance, conditional approval, public health.
Call to Action: Stay informed about the latest updates on COVID-19 vaccines and consult with your healthcare provider to make informed decisions about vaccination. Consult the for the most up-to-date information on COVID-19 vaccination.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Approval Comes With Strict Conditions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Deportations Loom Supreme Court Backs Trumps End To Tps For Some Venezuelans
May 20, 2025
Deportations Loom Supreme Court Backs Trumps End To Tps For Some Venezuelans
May 20, 2025 -
 Lsgs Must Win Game Srhs Marsh And Markram Fuel 206 Run Total
May 20, 2025
Lsgs Must Win Game Srhs Marsh And Markram Fuel 206 Run Total
May 20, 2025 -
 The Return Of Star Wars Battlefront A Detailed Look
May 20, 2025
The Return Of Star Wars Battlefront A Detailed Look
May 20, 2025 -
 The Lasting Scars Jenn Sterger Details The Emotional Toll Of The Brett Favre Scandal
May 20, 2025
The Lasting Scars Jenn Sterger Details The Emotional Toll Of The Brett Favre Scandal
May 20, 2025 -
 Fall Of Favre Director Discusses Depicting Brett Favres Complex Past
May 20, 2025
Fall Of Favre Director Discusses Depicting Brett Favres Complex Past
May 20, 2025
