Novavax COVID-19 Vaccine: FDA Approval, But With Significant Usage Constraints
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine: FDA Approval, but a Murky Future Ahead
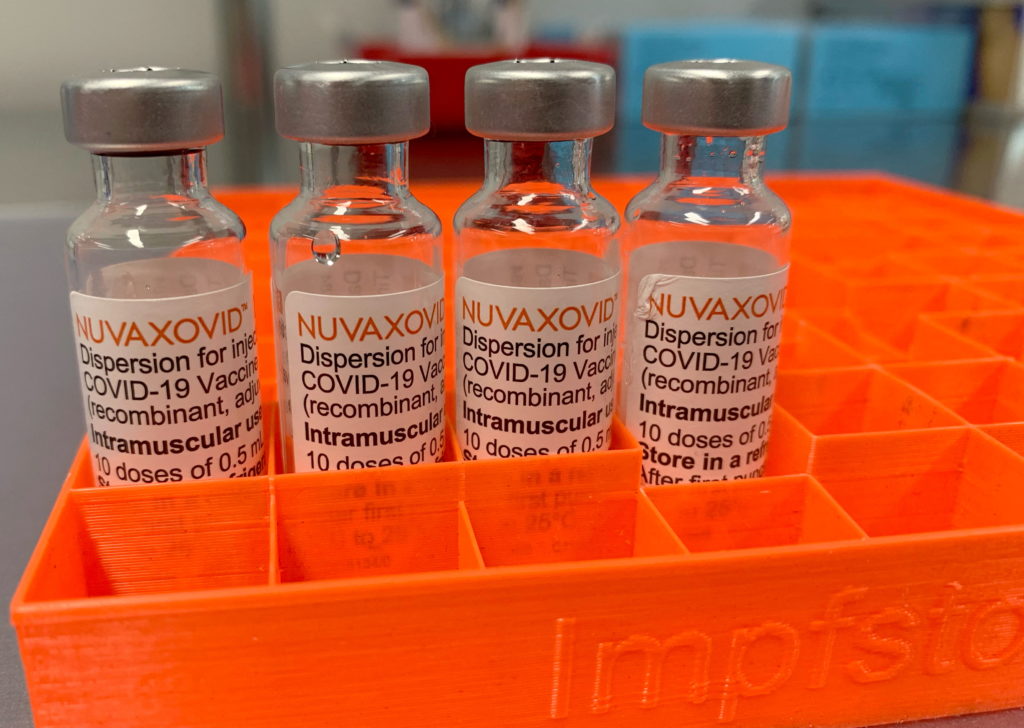
The Novavax COVID-19 vaccine, Nuvaxovid, finally received FDA approval in July 2023, a moment many anticipated would mark a significant shift in the pandemic response. However, the reality is far more nuanced. While approval is a major milestone for Novavax, significant usage constraints cast a shadow over its potential impact. This article delves into the complexities surrounding the vaccine's approval and the challenges it faces in a market already saturated with other COVID-19 vaccines.
Limited Demand in a Post-Pandemic Landscape:
The biggest hurdle facing Nuvaxovid is the drastically reduced demand for COVID-19 vaccines. The initial urgency of the pandemic has subsided, and widespread vaccination campaigns have already reached significant portions of the population. With other readily available vaccines, including mRNA vaccines like Pfizer-BioNTech and Moderna, and more established viral vector vaccines like Johnson & Johnson's Janssen, the market is essentially saturated. This makes it difficult for Novavax to establish a significant market share.
Protein Subunit Technology: A Double-Edged Sword:
Novavax employs a protein subunit technology, a well-established approach known for its generally good safety profile. This is a key selling point, particularly for individuals hesitant about mRNA vaccines. However, this technology is also associated with lower efficacy compared to mRNA vaccines, particularly against newer variants. This lower efficacy, coupled with the already reduced demand, poses a challenge to widespread adoption.
Logistical and Distribution Challenges:
The approval itself doesn't automatically translate into seamless distribution. The vaccine requires specific storage conditions, potentially adding complexity to its logistics and distribution compared to some competitor vaccines. This could further hinder its accessibility and uptake, particularly in areas with limited resources.
Who Might Benefit from Nuvaxovid?
Despite the challenges, Nuvaxovid still holds some potential. Certain groups might find it advantageous:
- Individuals hesitant about mRNA vaccines: For those who prefer a protein subunit vaccine due to concerns about mRNA technology, Nuvaxovid provides an alternative.
- Specific populations with limited access to other vaccines: In regions with limited access to other approved COVID-19 vaccines, Nuvaxovid might offer a crucial option.
The Future of Nuvaxovid:
The future of Nuvaxovid remains uncertain. While FDA approval is a crucial step, the vaccine faces significant obstacles in terms of market demand, competition, and logistical considerations. Its success will hinge on targeted marketing towards specific populations and overcoming the inherent challenges associated with entering a mature and competitive market. Further research into its efficacy against emerging variants will also be critical for its long-term viability. The coming months will be crucial in determining whether Nuvaxovid can find a niche and contribute meaningfully to global COVID-19 vaccination efforts.
Keywords: Novavax, COVID-19 vaccine, Nuvaxovid, FDA approval, vaccine efficacy, protein subunit vaccine, mRNA vaccine, vaccine hesitancy, vaccine distribution, pandemic response, public health
Call to Action (subtle): Stay informed about the latest developments in COVID-19 vaccination by following reputable sources such as the CDC and WHO websites.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Approval, But With Significant Usage Constraints. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Stream The Critically Acclaimed Wwi Film Featuring Daniel Craig Cillian Murphy And Tom Hardy
May 20, 2025
Stream The Critically Acclaimed Wwi Film Featuring Daniel Craig Cillian Murphy And Tom Hardy
May 20, 2025 -
 Confirmed New Peaky Blinders Series Coming Featuring A Key Departure
May 20, 2025
Confirmed New Peaky Blinders Series Coming Featuring A Key Departure
May 20, 2025 -
 Tariff Showdown Trump Tells Walmart To Absorb Increased Costs
May 20, 2025
Tariff Showdown Trump Tells Walmart To Absorb Increased Costs
May 20, 2025 -
 Live Score Updates Lsg Vs Srh Ipl 2025 Match Tensions Rise
May 20, 2025
Live Score Updates Lsg Vs Srh Ipl 2025 Match Tensions Rise
May 20, 2025 -
 Freaky Friday Reunion Jamie Lee Curtis Speaks On Lindsay Lohan Friendship
May 20, 2025
Freaky Friday Reunion Jamie Lee Curtis Speaks On Lindsay Lohan Friendship
May 20, 2025
