Novavax COVID-19 Vaccine: FDA Approval And Usage Restrictions Explained
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine: FDA Approval, Usage, and Restrictions Explained
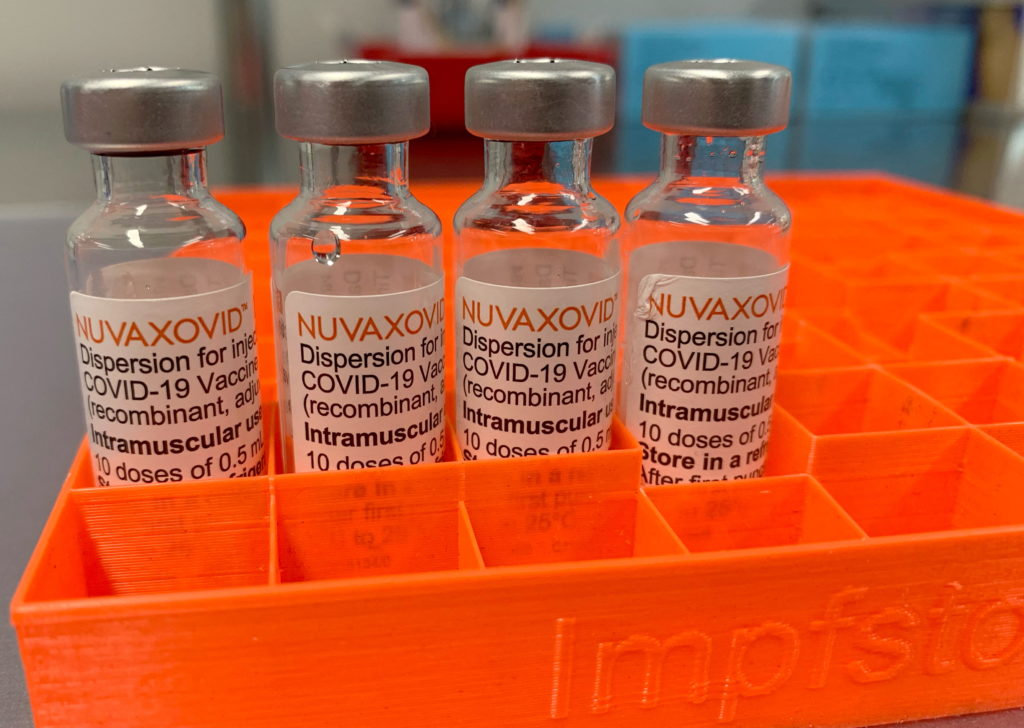
The Novavax COVID-19 vaccine, Nuvaxovid (also known as Covovax internationally), represents a different approach to COVID-19 immunization compared to mRNA vaccines like Pfizer-BioNTech and Moderna. Its approval by the FDA marked a significant milestone, offering an alternative for individuals hesitant about mRNA technology. However, understanding its usage and any associated restrictions is crucial. This article will break down the key details.
What is the Novavax COVID-19 Vaccine?
Unlike mRNA vaccines that utilize messenger RNA to instruct cells to produce a viral protein, the Novavax vaccine uses a more traditional approach. It's a protein subunit vaccine, meaning it contains purified fragments of the SARS-CoV-2 virus's spike protein. This protein is produced in insect cells and is then formulated with an adjuvant, which enhances the immune response. This method has been used for years in other vaccines, potentially making it more appealing to those concerned about the novelty of mRNA technology.
FDA Approval and Authorization:
The Novavax vaccine received Emergency Use Authorization (EUA) from the FDA in July 2022 for individuals 18 years and older. This authorization was later followed by full FDA approval in July 2023. This full approval signifies a rigorous review process validating the vaccine's safety and effectiveness.
Effectiveness and Safety:
Clinical trials demonstrated the Novavax vaccine to be highly effective in preventing COVID-19. While efficacy might slightly vary compared to mRNA vaccines, it still provides substantial protection against severe illness, hospitalization, and death. As with any vaccine, side effects are possible, but generally mild and temporary. Common side effects include:
- Pain, redness, and swelling at the injection site
- Fatigue
- Headache
- Muscle aches
- Joint pain
- Nausea
Serious side effects are rare. Anyone experiencing severe or unusual symptoms should seek immediate medical attention.
Usage Restrictions and Considerations:
While generally safe and effective, some restrictions and considerations surround the Novavax vaccine:
- Age Restrictions: Initially, the EUA and subsequent full approval were for individuals 18 years and older. Further studies may expand its use to younger age groups.
- Allergies: Individuals with a known history of severe allergic reactions to any component of the vaccine should not receive it. It's crucial to inform your healthcare provider about any allergies before vaccination.
- Pregnancy and Breastfeeding: While data on pregnant and breastfeeding individuals is limited, the CDC generally considers the benefits of vaccination to outweigh potential risks. Consult your healthcare provider for personalized advice.
- Immunocompromised Individuals: The vaccine's effectiveness might be reduced in immunocompromised individuals. Consult with your doctor to discuss the best vaccination strategy.
Where to Get the Novavax Vaccine:
The availability of the Novavax vaccine may vary depending on location. Check with your healthcare provider, local pharmacies, or your state's health department to find vaccination sites offering Nuvaxovid. You can also use online search engines to locate nearby vaccination centers offering the Novavax vaccine.
Conclusion:
The Novavax COVID-19 vaccine provides a valuable alternative for individuals seeking a protein subunit vaccine. While its effectiveness and safety profile are generally positive, understanding its usage restrictions and consulting with a healthcare professional before vaccination remains crucial. Staying informed about COVID-19 vaccines is vital for making informed decisions about your health and the health of your community. Remember to consult your doctor before making any vaccination decisions.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Approval And Usage Restrictions Explained. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 2025s Largest Solar Flare Impact Assessment And Radio Blackout Analysis
May 20, 2025
2025s Largest Solar Flare Impact Assessment And Radio Blackout Analysis
May 20, 2025 -
 Marsh Markram Power Srh To 206 Lucknows Must Win Game
May 20, 2025
Marsh Markram Power Srh To 206 Lucknows Must Win Game
May 20, 2025 -
 Los Angeles Federal Courthouse Sale A Rapid Transaction In The Works
May 20, 2025
Los Angeles Federal Courthouse Sale A Rapid Transaction In The Works
May 20, 2025 -
 From Labs To Lives The Real World Benefits Of American Medical And Scientific Research
May 20, 2025
From Labs To Lives The Real World Benefits Of American Medical And Scientific Research
May 20, 2025 -
 Overcompensating A Coming Out Comedy Thats Both Funny And Thought Provoking
May 20, 2025
Overcompensating A Coming Out Comedy Thats Both Funny And Thought Provoking
May 20, 2025
