Novavax COVID-19 Vaccine: FDA Approval And The Specific Use Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine: FDA Approval, Use Restrictions, and What You Need to Know
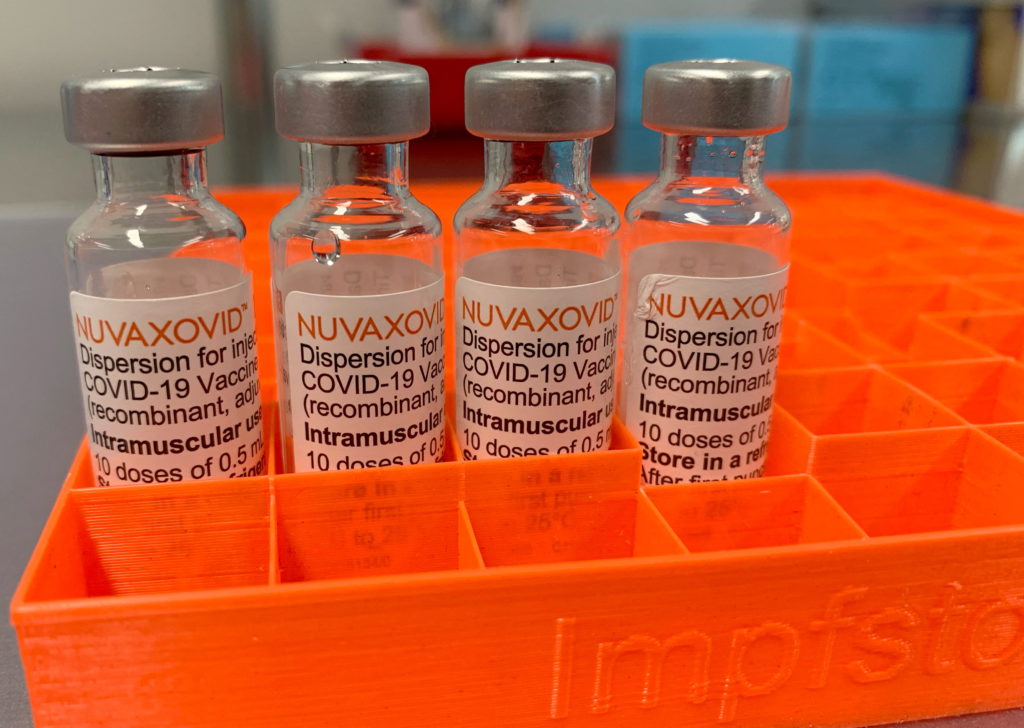
The arrival of the Novavax COVID-19 vaccine, Nuvaxovid, marked a significant development in the global fight against the pandemic. Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, Novavax utilizes a more traditional protein subunit technology. This difference sparked considerable interest, but also raised questions regarding its FDA approval and specific use restrictions. Let's delve into the details.
FDA Approval and Authorization:
The Novavax vaccine received Emergency Use Authorization (EUA) from the FDA in July 2022 for individuals 18 years and older. This authorization was a crucial step, making the vaccine available to the public. It's important to distinguish between EUA and full FDA approval. While EUA allows for the use of a vaccine during a public health emergency, full approval follows a more rigorous review process and signifies a higher level of confidence in the vaccine's safety and efficacy. As of October 2023, Novavax retains its EUA status; however, the FDA continually monitors its safety profile and efficacy data. [Link to FDA website on Novavax approval].
Specific Use Restrictions and Considerations:
While generally safe and effective, the Novavax vaccine has specific use restrictions:
- Age: Initially, the EUA was limited to adults 18 years and older. Further studies may expand this age range in the future.
- Allergies: Individuals with a history of severe allergic reactions to any component of the vaccine should avoid it. This includes a history of severe allergic reactions to previous vaccines or other medications. It's crucial to discuss any allergies with your healthcare provider before receiving the vaccination.
- Pre-existing Conditions: While generally safe, individuals with pre-existing conditions should consult their physician to assess any potential risks or interactions. This is standard procedure for any vaccination.
- Pregnancy and Breastfeeding: Data on the use of Novavax during pregnancy and breastfeeding is still emerging. Pregnant or breastfeeding individuals should discuss the benefits and risks with their healthcare providers before making a decision.
How Novavax Differs and Potential Advantages:
Novavax's protein subunit technology differs significantly from the mRNA vaccines. This difference could be advantageous for certain individuals:
- Vaccine Hesitancy: Some individuals hesitant about mRNA technology might find the more traditional protein subunit approach more acceptable.
- Reduced Side Effects: Some individuals report fewer side effects with the Novavax vaccine compared to mRNA vaccines, although this can vary significantly from person to person. Common side effects include pain at the injection site, fatigue, headache, muscle aches, and joint pain. [Link to study comparing side effects of different COVID-19 vaccines].
The Bottom Line:
The Novavax COVID-19 vaccine offers a valuable alternative in the fight against COVID-19. While it has received EUA from the FDA, specific use restrictions apply. It’s essential to consult with your healthcare provider to determine if the Novavax vaccine is the right choice for you based on your individual health history and circumstances. Staying informed about vaccine updates and safety information is crucial for making informed decisions about your health. [Link to CDC website on COVID-19 vaccines].
Disclaimer: This article provides general information and should not be considered medical advice. Always consult with a healthcare professional before making any decisions related to your health or treatment.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Approval And The Specific Use Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Solo Levelings Award Winning Success A Look At The Future
May 21, 2025
Solo Levelings Award Winning Success A Look At The Future
May 21, 2025 -
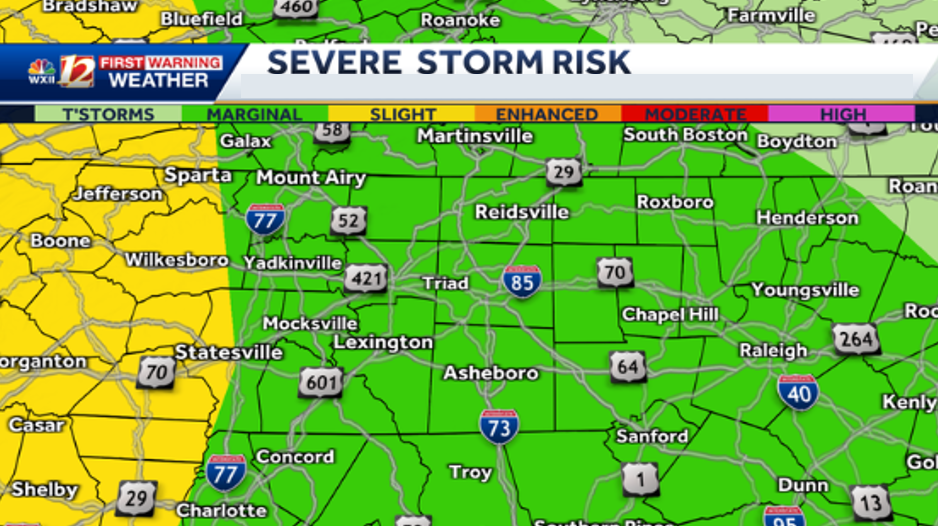 Severe Weather Alert Heavy Rain And Storms To Hit North Carolina Tonight
May 21, 2025
Severe Weather Alert Heavy Rain And Storms To Hit North Carolina Tonight
May 21, 2025 -
 160 Japanese Companies Vie For Higher Value Through Nature Conservation 13 Industry Guidelines Unveiled
May 21, 2025
160 Japanese Companies Vie For Higher Value Through Nature Conservation 13 Industry Guidelines Unveiled
May 21, 2025 -
 A Quieter Wes Anderson Exploring The Understated Power Of The Phoenician Scheme
May 21, 2025
A Quieter Wes Anderson Exploring The Understated Power Of The Phoenician Scheme
May 21, 2025 -
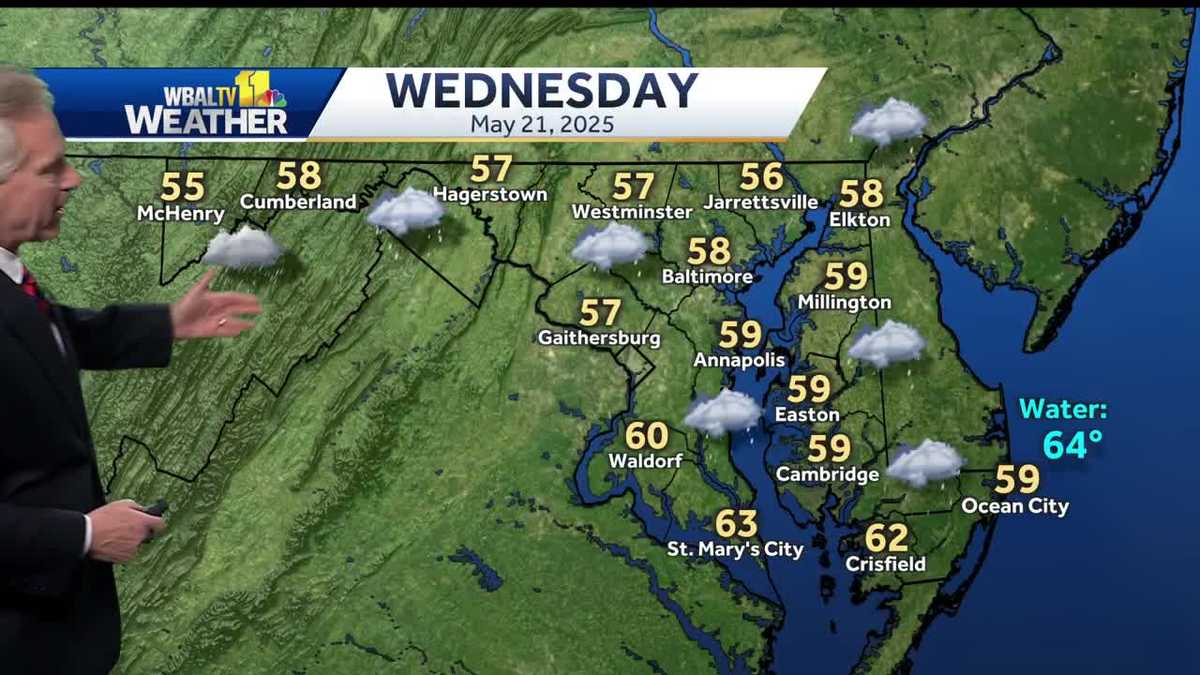 Regional Weather Forecast Rain And Cold To Continue Through Wednesday
May 21, 2025
Regional Weather Forecast Rain And Cold To Continue Through Wednesday
May 21, 2025
