Novavax COVID-19 Vaccine: FDA Approval And Key Restrictions Explained
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine: FDA Approval, Key Restrictions, and What You Need to Know
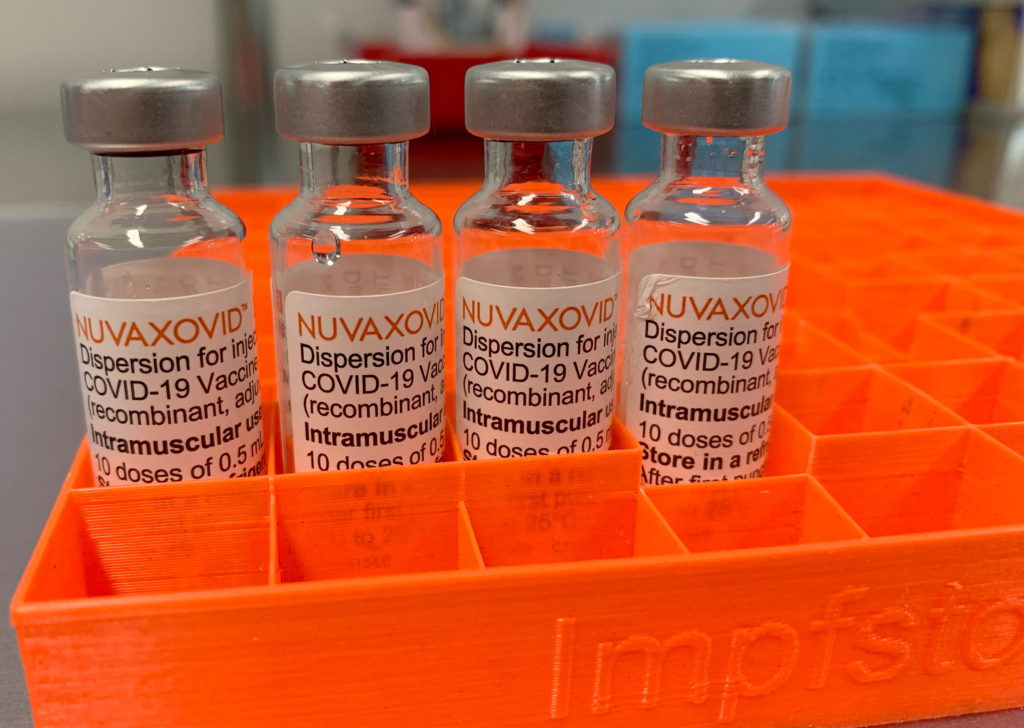
The Novavax COVID-19 vaccine, Nuvaxovid (also known as Covovax internationally), has received FDA approval, marking a significant development in the fight against the pandemic. However, its journey to widespread adoption isn't without its nuances. This article breaks down the FDA approval, explains the key restrictions, and answers common questions surrounding this protein subunit vaccine.
FDA Approval and Authorization:
The FDA granted Novavax's COVID-19 vaccine Emergency Use Authorization (EUA) in July 2022, allowing its use in individuals 12 years and older. A crucial step forward came in July 2023 when the FDA transitioned the authorization to full approval for individuals 18 years and older. This full approval signifies a rigorous review process and increased confidence in the vaccine's safety and efficacy profile compared to the EUA. This is a significant difference for many, and the transition from EUA to full FDA approval provides reassurance to those hesitant about receiving a vaccine under an emergency authorization. You can find more details on the FDA's website regarding the approval process and documentation. [Link to FDA website]
Key Restrictions and Considerations:
While fully approved for adults, some key restrictions and considerations surround the Novavax vaccine:
- Age Restriction (18+ for Full Approval): While initially authorized for ages 12 and up under EUA, the full FDA approval currently only applies to individuals 18 years and older. Clinical trials for younger age groups are ongoing.
- Specific Ingredients: Novavax utilizes a protein subunit technology, differing from mRNA vaccines like Pfizer-BioNTech and Moderna. This may be a crucial factor for those with allergies or concerns about mRNA technology. However, it's vital to discuss any allergies or concerns with a healthcare professional before vaccination.
- Efficacy Compared to mRNA Vaccines: While effective, studies have shown Novavax's efficacy might be slightly lower than that of mRNA vaccines, particularly against certain variants. However, it still provides substantial protection against severe illness, hospitalization, and death. [Link to relevant study/research]
- Storage and Handling: Nuvaxovid has specific storage and handling requirements, impacting its distribution and availability compared to mRNA vaccines. These requirements contribute to potential logistical challenges.
Understanding the Protein Subunit Technology:
Unlike mRNA vaccines that introduce mRNA to instruct cells to produce the virus's spike protein, Novavax uses a different approach. It uses purified fragments of the spike protein itself to trigger an immune response. This method is considered more traditional and might appeal to those hesitant about mRNA technology. This technology has been used in other vaccines successfully for years.
Who Should Consider the Novavax Vaccine?
The Novavax vaccine provides a valuable option for individuals:
- Hesitant about mRNA vaccines: The different technology may be a deciding factor for those with concerns about mRNA vaccines.
- Experiencing side effects with other vaccines: While side effects are possible, they are typically mild and less common than with some other vaccines.
- Seeking a protein-subunit option: For those who prefer this established vaccine technology, Novavax presents a clear choice.
Conclusion:
The FDA approval of the Novavax COVID-19 vaccine is a positive step, offering an alternative approach to vaccination. Understanding its specific restrictions, efficacy, and technological differences compared to mRNA vaccines is crucial for making informed decisions. Always consult with your healthcare provider to determine the best vaccination strategy for your individual circumstances. Remember to stay updated on the latest information from reputable sources like the CDC and FDA.
Call to Action: Talk to your doctor today to learn more about the Novavax vaccine and whether it's the right choice for you. [Link to CDC website or relevant health authority]

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine: FDA Approval And Key Restrictions Explained. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Big Changes Ahead New Peaky Blinders Series Officially Announced
May 20, 2025
Big Changes Ahead New Peaky Blinders Series Officially Announced
May 20, 2025 -
 Taylor Jenkins Reids Publishing Empire Strategies And Insights
May 20, 2025
Taylor Jenkins Reids Publishing Empire Strategies And Insights
May 20, 2025 -
 Environmental Threats To Pregnancy Understanding Climate Changes Role
May 20, 2025
Environmental Threats To Pregnancy Understanding Climate Changes Role
May 20, 2025 -
 La Tanya Richardson Jackson Her Unbreakable Bond With Samuel L Jackson
May 20, 2025
La Tanya Richardson Jackson Her Unbreakable Bond With Samuel L Jackson
May 20, 2025 -
 Confirmed A New Peaky Blinders Series Is In The Works Expect The Unexpected
May 20, 2025
Confirmed A New Peaky Blinders Series Is In The Works Expect The Unexpected
May 20, 2025
