Novavax COVID-19 Vaccine Approved By FDA With Unusual Limitations
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Approved by FDA, but with Unusual Limitations
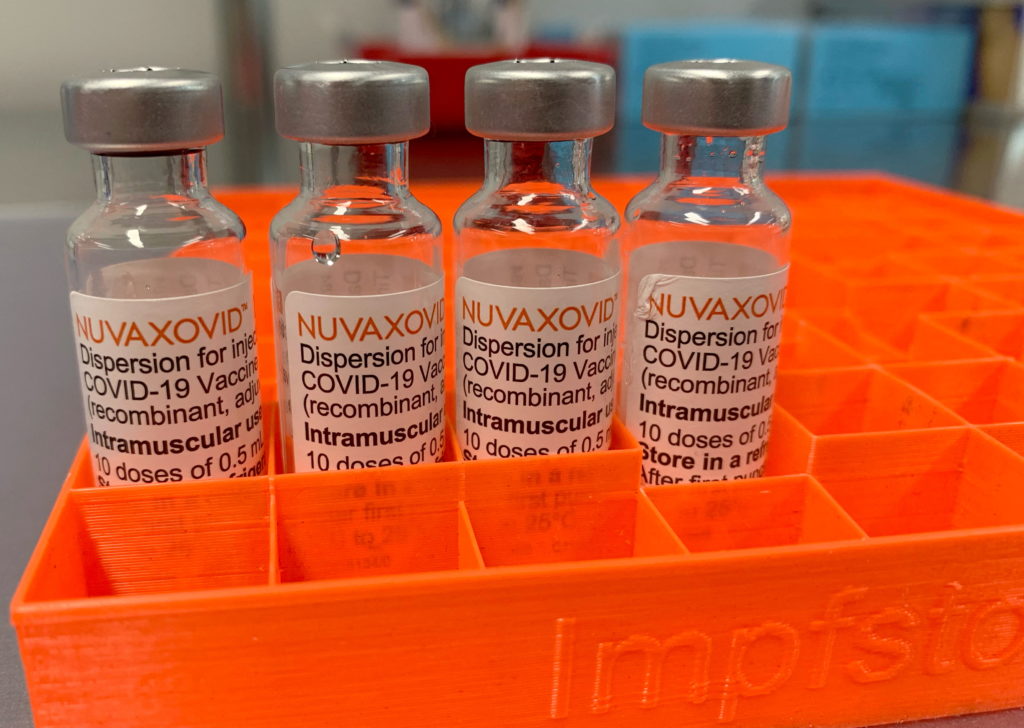
The FDA has finally granted approval to Novavax's COVID-19 vaccine, Nuvaxovid, but its journey to market has been anything but straightforward. The approval, announced [insert date], comes with several unusual limitations, raising questions about its future role in the ongoing fight against the pandemic. While many hail the approval as broadening vaccine options, the caveats attached warrant a closer look.
A Long and Winding Road to FDA Approval:
Novavax's vaccine, unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, utilizes a more traditional protein subunit technology. This technology has been around for decades and is considered by some to be safer, potentially appealing to vaccine-hesitant individuals. However, delays in manufacturing and clinical trial results significantly hampered its rollout. This lengthy process has resulted in a vaccine entering the market at a time when COVID-19 infection rates are significantly lower than previous peaks.
The Unusual Limitations:
The FDA's approval comes with several notable limitations:
-
Limited Age Range: Unlike other authorized COVID-19 vaccines, Nuvaxovid is currently only approved for individuals 18 years of age and older. This contrasts with the wider age ranges covered by mRNA vaccines. This restriction limits its potential impact on overall vaccination rates.
-
Conditional Approval: The approval is conditional, meaning that Novavax must continue to meet certain benchmarks regarding safety and efficacy. This is not uncommon for newly approved vaccines, but it highlights the ongoing scrutiny surrounding Nuvaxovid's performance.
-
Lower Efficacy Rates in Some Trials: While the vaccine demonstrated efficacy in clinical trials, the reported rates were lower than those observed with the mRNA vaccines. This difference has contributed to some questioning the vaccine's overall effectiveness in the current pandemic landscape.
The Role of Nuvaxovid in the Future:
Despite the limitations, the approval of Nuvaxovid could still hold significance. Its protein subunit technology may offer advantages for specific populations, particularly those with pre-existing conditions or a history of adverse reactions to other vaccines. Furthermore, the availability of a diverse range of vaccines can enhance vaccination efforts globally, especially in regions with limited access to mRNA vaccines. [Link to WHO information on global vaccine access]
Concerns and Future Research:
Several questions remain. Will the relatively late approval and limited age range significantly impact its uptake? Will further research be needed to address the lower efficacy rates observed in some trials? The long-term safety profile and efficacy against emerging variants also require ongoing monitoring. [Link to CDC data on COVID-19 variants]
Conclusion:
The FDA's approval of the Novavax COVID-19 vaccine marks a significant milestone, but the accompanying limitations paint a complex picture. While it offers a different vaccine technology and might appeal to some hesitant individuals, its late entry into the market and the conditional approval raise questions about its practical impact on the current pandemic situation. The vaccine’s future success will depend on overcoming these hurdles and addressing the remaining uncertainties surrounding its efficacy and long-term safety. Further research and monitoring will be crucial in determining its ultimate role in the ongoing battle against COVID-19.
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, protein subunit vaccine, vaccine efficacy, vaccine safety, conditional approval, mRNA vaccines, pandemic, vaccination, COVID-19 variants.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Approved By FDA With Unusual Limitations. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Deciphering A Gleason Score Of 9 Implications And Treatment Options
May 20, 2025
Deciphering A Gleason Score Of 9 Implications And Treatment Options
May 20, 2025 -
 World War I Epic Featuring Daniel Craig Cillian Murphy And Tom Hardy Available To Stream
May 20, 2025
World War I Epic Featuring Daniel Craig Cillian Murphy And Tom Hardy Available To Stream
May 20, 2025 -
 Get Ready For Helldivers 2 Masters Of Ceremony Warbond Drop May 15th
May 20, 2025
Get Ready For Helldivers 2 Masters Of Ceremony Warbond Drop May 15th
May 20, 2025 -
 Breaking Trump Initiates Russia Ukraine Peace Talks
May 20, 2025
Breaking Trump Initiates Russia Ukraine Peace Talks
May 20, 2025 -
 Balis Tourism Crisis A Plea For Global Collaboration On Safety And Conduct
May 20, 2025
Balis Tourism Crisis A Plea For Global Collaboration On Safety And Conduct
May 20, 2025
