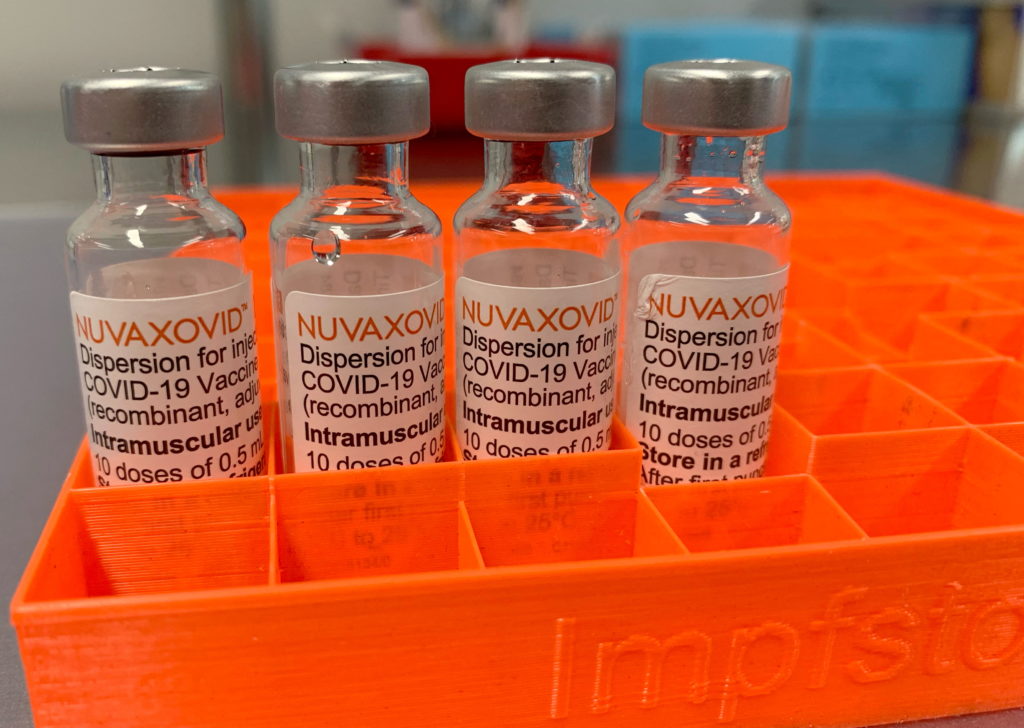
Novavax COVID-19 Vaccine Approved By FDA, But With Usage Limitations
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Novavax COVID-19 Vaccine Approved by FDA, But with Usage Limitations
The FDA has finally granted approval to Novavax's COVID-19 vaccine, Nuvaxovid, but with significant usage limitations. This landmark decision, while welcomed by some, is tempered by the vaccine's restricted application compared to already established COVID-19 vaccines like Pfizer-BioNTech and Moderna. The approval marks a significant step in the fight against the pandemic, but the nuances of its limited use raise important questions.
A Long-Awaited Approval, but with Caveats
The approval of Nuvaxovid, a protein subunit vaccine, offers a different technological approach to COVID-19 prevention compared to the mRNA vaccines currently dominating the market. Many have eagerly awaited this alternative, hoping it might address vaccine hesitancy among certain populations. However, the FDA's approval comes with crucial limitations:
- Limited Authorization: The FDA's Emergency Use Authorization (EUA) has been replaced with full approval, but only for individuals 18 years of age and older. This contrasts with the broader age ranges covered by the mRNA vaccines. Further research and trials are needed to extend the approval to younger age groups.
- Conditional Approval: The approval is conditional upon Novavax meeting ongoing post-market requirements, including further data collection and analysis. This means the approval isn't a definitive stamp of unconditional acceptance, leaving room for potential future adjustments.
- Efficacy Concerns: While effective, the vaccine’s efficacy data, particularly against newer variants, might not be as robust as the mRNA counterparts. The FDA's decision acknowledges this, highlighting the need for continued monitoring and research.
Why the Limitations?
The FDA's decision to approve Nuvaxovid with limitations reflects a cautious approach. While the protein subunit technology has a long history of safe and effective use in other vaccines, its efficacy against emerging COVID-19 variants requires further scrutiny. The limited age range authorization also underscores the importance of extensive clinical trial data before widespread deployment among younger populations. This measured approach prioritizes safety and efficacy, aligning with the FDA's rigorous standards.
What Does this Mean for the Future of COVID-19 Vaccination?
The approval of the Novavax vaccine presents both opportunities and challenges. Its alternative technology could help bridge the gap in vaccine hesitancy, providing a choice for individuals who prefer a non-mRNA vaccine. However, the limitations imposed by the FDA highlight the ongoing need for robust research and monitoring of all COVID-19 vaccines.
Looking Ahead:
The FDA's approval of the Novavax COVID-19 vaccine is a significant development, yet the conditional nature of the approval and the limitations on its use underscore the dynamic nature of the pandemic and the ongoing need for scientific rigor. Further research is crucial to expand the vaccine’s applicability and bolster its effectiveness against emerging variants. The long-term impact of Nuvaxovid’s introduction to the market remains to be seen, but its arrival offers a potentially valuable new tool in the fight against COVID-19. For the most up-to-date information on COVID-19 vaccines, consult your doctor or visit the .
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, protein subunit vaccine, mRNA vaccine, vaccine hesitancy, vaccine efficacy, COVID-19 variants, pandemic, public health, vaccine safety, coronavirus, immunization.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Novavax COVID-19 Vaccine Approved By FDA, But With Usage Limitations. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Daniel Craig Cillian Murphy And Tom Hardy Star In This Must See Wwi Film
May 20, 2025
Daniel Craig Cillian Murphy And Tom Hardy Star In This Must See Wwi Film
May 20, 2025 -
 Helldivers 2 Warbond Event Masters Of Ceremony Rewards On May 15th
May 20, 2025
Helldivers 2 Warbond Event Masters Of Ceremony Rewards On May 15th
May 20, 2025 -
 The Lasting Scars Jenn Sterger Details The Emotional Toll Of The Brett Favre Scandal
May 20, 2025
The Lasting Scars Jenn Sterger Details The Emotional Toll Of The Brett Favre Scandal
May 20, 2025 -
 Us Supreme Court Greenlights End To Tps For Some Venezuelans What This Means
May 20, 2025
Us Supreme Court Greenlights End To Tps For Some Venezuelans What This Means
May 20, 2025 -
 Exclusive Jamie Lee Curtis On Maintaining Her Bond With Lindsay Lohan Years After Freaky Friday
May 20, 2025
Exclusive Jamie Lee Curtis On Maintaining Her Bond With Lindsay Lohan Years After Freaky Friday
May 20, 2025
