New Alzheimer's Drug: Home Injection Option Approved
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Revolutionary Alzheimer's Drug Approved: Home Injection Offers New Hope
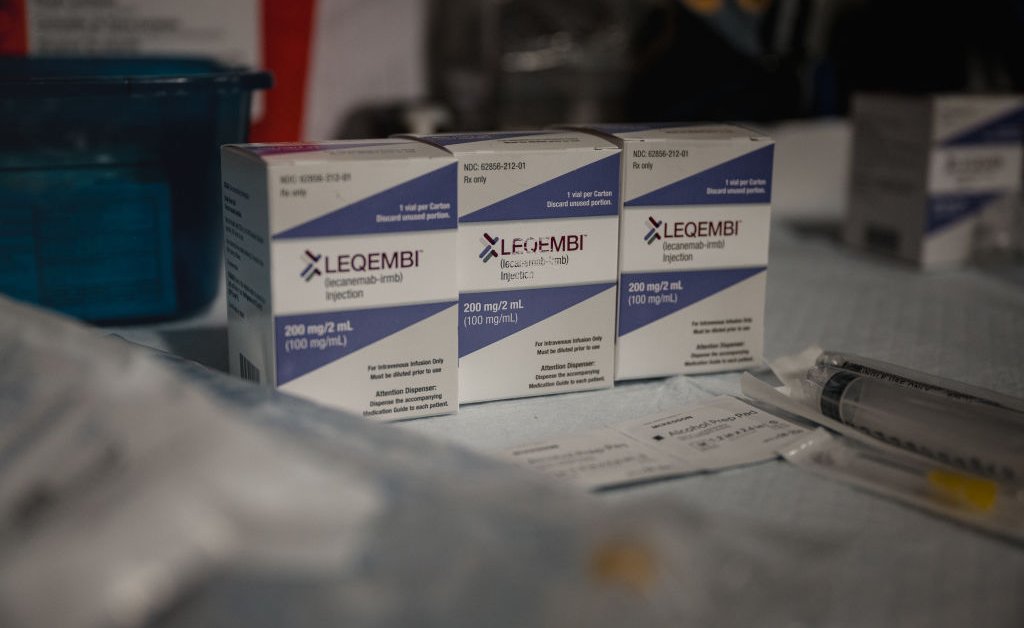
A groundbreaking new Alzheimer's drug, Leqembi (lecanemab), has received full FDA approval, marking a significant leap forward in the fight against this devastating disease. Crucially, the approval includes the option for home administration via injection, potentially improving patient access and quality of life. This development represents a monumental shift in Alzheimer's treatment, offering a new path forward for millions affected by this debilitating condition.
The approval of Leqembi, following its accelerated approval in January 2023, is based on robust clinical trial data demonstrating its ability to slow cognitive decline in early-stage Alzheimer's disease. This is a significant step beyond previous treatments that only managed symptoms. The drug targets amyloid plaques, a hallmark characteristic of Alzheimer's, effectively reducing their buildup in the brain.
How Leqembi Works and Its Benefits
Leqembi is a monoclonal antibody that works by binding to and clearing amyloid beta plaques, which are believed to contribute significantly to the cognitive decline associated with Alzheimer's. Clinical trials showed that Leqembi slowed cognitive decline by 27% compared to a placebo. While not a cure, this slowing of the disease progression offers invaluable time and improved quality of life for patients and their families.
- Improved Cognitive Function: Studies indicate a noticeable improvement in cognitive functions like memory and thinking.
- Slower Disease Progression: Leqembi demonstrably slows the rate at which Alzheimer's symptoms worsen.
- Home Injection Convenience: The approval of home administration significantly enhances accessibility and reduces the burden on patients and caregivers.
The Significance of Home Injection
The ability to self-administer Leqembi at home via subcutaneous injection is a game-changer. This eliminates the need for frequent trips to clinics or hospitals, making treatment more convenient and less disruptive to patients' daily lives. This is particularly crucial for individuals living in rural areas or those with limited mobility. However, proper training on the injection technique will be essential, and patients will likely receive guidance from healthcare professionals initially.
Potential Side Effects and Considerations
While Leqembi offers significant hope, it's important to acknowledge potential side effects. The most common side effects include infusion-related reactions (though less likely with home injections), brain swelling (ARIA-E), and brain bleeding (ARIA-H). These risks will be carefully monitored, and patients should discuss any concerns with their healthcare provider. Early detection and diagnosis remain crucial for effective treatment and to maximize the benefits of Leqembi.
The Future of Alzheimer's Treatment
The FDA's full approval of Leqembi with the home injection option represents a remarkable achievement in the ongoing battle against Alzheimer's disease. It underscores the importance of continued research and development in this field. This landmark decision offers new hope for patients and their families, paving the way for a future with more effective treatments and improved quality of life for those affected by this devastating condition. Further research is underway to explore the drug's effectiveness in later stages of the disease and to develop even more innovative therapies.
For more information on Alzheimer's disease, diagnosis, and treatment options, please consult your doctor or visit the Alzheimer's Association website: [link to Alzheimer's Association]

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on New Alzheimer's Drug: Home Injection Option Approved. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 World Cup Ticket Sales How To Secure Your Seats And Your Wallet
Sep 09, 2025
World Cup Ticket Sales How To Secure Your Seats And Your Wallet
Sep 09, 2025 -
 Faith Transition A Christian Influencer Shares Why They Stopped Going To Church
Sep 09, 2025
Faith Transition A Christian Influencer Shares Why They Stopped Going To Church
Sep 09, 2025 -
 Commentary Debut Jj Watt Shows Off Edgy New Hairstyle
Sep 09, 2025
Commentary Debut Jj Watt Shows Off Edgy New Hairstyle
Sep 09, 2025 -
 Lower Mortgage Rates Predicted Analyzing The September Fed Rate Cuts Impact
Sep 09, 2025
Lower Mortgage Rates Predicted Analyzing The September Fed Rate Cuts Impact
Sep 09, 2025 -
 World Cup Tickets Your Guide To The First Presale This Week
Sep 09, 2025
World Cup Tickets Your Guide To The First Presale This Week
Sep 09, 2025
Latest Posts
-
 Home Administration Of Alzheimers Medication A New Era Of Treatment
Sep 09, 2025
Home Administration Of Alzheimers Medication A New Era Of Treatment
Sep 09, 2025 -
 From Gridiron To Green Screen J J Watts Transition To Cbs And The Lessons From Romo
Sep 09, 2025
From Gridiron To Green Screen J J Watts Transition To Cbs And The Lessons From Romo
Sep 09, 2025 -
 Sherrone Moore Suspended Biff Poggi Takes The Helm For Michigan
Sep 09, 2025
Sherrone Moore Suspended Biff Poggi Takes The Helm For Michigan
Sep 09, 2025 -
 Wall Street Rallies S And P 500 Nasdaq And Dow Higher Ahead Of Inflation Data
Sep 09, 2025
Wall Street Rallies S And P 500 Nasdaq And Dow Higher Ahead Of Inflation Data
Sep 09, 2025 -
 Supreme Court Upholds Trump Era Immigration Raids Live Updates
Sep 09, 2025
Supreme Court Upholds Trump Era Immigration Raids Live Updates
Sep 09, 2025
