Navigating The Complexities Of U.S. Tariffs For Swiss Pharmaceutical Manufacturers

Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Navigating the Complexities of U.S. Tariffs for Swiss Pharmaceutical Manufacturers
The U.S. market, while lucrative for Swiss pharmaceutical manufacturers, presents a complex landscape of tariffs and regulations. Successfully exporting to the United States requires a thorough understanding of these complexities to avoid costly delays and penalties. This article will guide Swiss pharmaceutical companies through the intricacies of navigating the U.S. tariff system.
Understanding the U.S. Tariff Structure
The United States utilizes a Harmonized Tariff Schedule (HTS) to classify imported goods and determine applicable tariffs. This system, based on the World Customs Organization's (WCO) Harmonized System, is incredibly detailed. Even slight variations in product composition or intended use can significantly impact the assigned HTS code and, consequently, the tariff rate. Pharmaceutical products, with their diverse formulations and specialized components, require particularly careful classification. Incorrect classification can lead to delays, fines, and even the rejection of shipments.
Key Considerations for Swiss Pharmaceutical Exporters:
-
HTS Code Determination: Accurately determining the correct HTS code is paramount. Swiss pharmaceutical manufacturers should consult with customs brokers experienced in pharmaceutical regulations or utilize resources like the U.S. International Trade Commission's website to ensure accurate classification. Incorrect classification can lead to significant financial repercussions.
-
Country of Origin Rules: Understanding country-of-origin rules is crucial for determining tariff eligibility. The rules dictate where a product is considered to be manufactured, taking into account the origin of components and manufacturing processes. Complex supply chains common in the pharmaceutical industry necessitate careful consideration of these regulations.
-
Free Trade Agreements (FTAs): While the U.S. doesn't have a comprehensive FTA with Switzerland, understanding any potential preferential tariff treatments under existing agreements is crucial. Even seemingly minor reductions can translate to significant cost savings over large export volumes.
-
FDA Regulations: Beyond tariffs, the U.S. Food and Drug Administration (FDA) imposes stringent regulations on pharmaceutical products. Meeting FDA requirements is essential for market access, irrespective of tariff considerations. Navigating both tariff and regulatory compliance simultaneously requires a strategic approach. This often necessitates engaging specialized consultants familiar with both U.S. Customs and Border Protection (CBP) and FDA regulations.
-
Duty Drawback: Swiss pharmaceutical companies should explore the possibility of duty drawback programs. These programs allow for refunds or reductions of duties paid on imported goods that are subsequently used in the production of exported products. Proper documentation and adherence to specific program rules are essential to take advantage of these potential savings.
Minimizing Tariff Risks:
-
Consult with Customs Brokers: Engaging experienced customs brokers specializing in pharmaceutical imports is highly recommended. They can navigate the complexities of HTS code classification, country of origin rules, and other regulatory hurdles.
-
Proactive Planning: Thorough pre-export planning, including accurate documentation and meticulous attention to detail, minimizes the risk of delays and penalties.
-
Staying Updated: The U.S. tariff landscape is dynamic. Swiss pharmaceutical manufacturers must remain updated on changes in regulations, HTS codes, and FTA agreements to maintain compliance. Regularly checking the CBP and FDA websites is essential.
Conclusion:
Exporting pharmaceutical products from Switzerland to the United States presents significant challenges due to the complexities of U.S. tariffs and regulations. By understanding the nuances of HTS codes, country of origin rules, and leveraging resources like customs brokers and up-to-date information, Swiss pharmaceutical manufacturers can mitigate risks and successfully tap into this lucrative market. Proactive planning and a strong understanding of both tariff and regulatory landscapes are key to achieving long-term success in the U.S. market.
Call to Action: For further assistance navigating the complexities of U.S. tariffs, consider consulting with a specialist in international trade law or a customs broker experienced in pharmaceutical regulations.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Navigating The Complexities Of U.S. Tariffs For Swiss Pharmaceutical Manufacturers. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 South Africas Proteas Two New Faces In The Test Squad
Jul 07, 2025
South Africas Proteas Two New Faces In The Test Squad
Jul 07, 2025 -
 Hoult On Rosenbaum Lex Luthors Unexpected Life Lessons
Jul 07, 2025
Hoult On Rosenbaum Lex Luthors Unexpected Life Lessons
Jul 07, 2025 -
 Oregon State Football Player Ranked Among Top 100 In Ea Sports College Football Game
Jul 07, 2025
Oregon State Football Player Ranked Among Top 100 In Ea Sports College Football Game
Jul 07, 2025 -
 Nicholas Hoult Reveals Smallville Stars Career Guidance
Jul 07, 2025
Nicholas Hoult Reveals Smallville Stars Career Guidance
Jul 07, 2025 -
 Ea Sports College Football 2026 Who Are Iowas Highest Rated Players
Jul 07, 2025
Ea Sports College Football 2026 Who Are Iowas Highest Rated Players
Jul 07, 2025
Latest Posts
-
 Trumps Tax Bill Increased Hunger Concerns For Iowa Food Pantries
Jul 07, 2025
Trumps Tax Bill Increased Hunger Concerns For Iowa Food Pantries
Jul 07, 2025 -
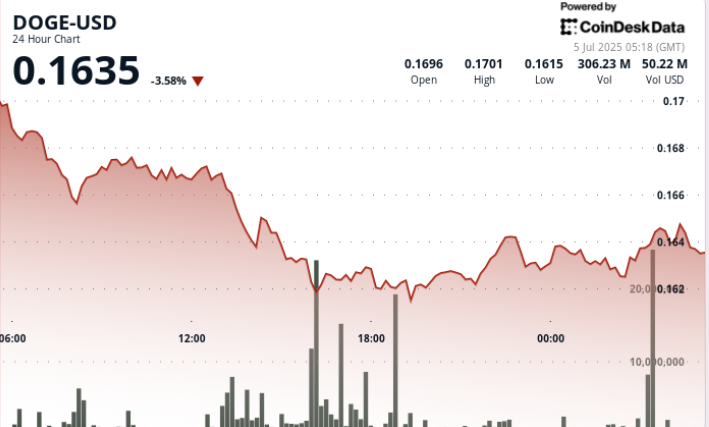 Dogecoin Price Holds Steady 0 16 Support Level Key For Bulls
Jul 07, 2025
Dogecoin Price Holds Steady 0 16 Support Level Key For Bulls
Jul 07, 2025 -
 Israeli Air Force Targets Yemeni Ports And Galaxy Leader Vessel Idf Statement
Jul 07, 2025
Israeli Air Force Targets Yemeni Ports And Galaxy Leader Vessel Idf Statement
Jul 07, 2025 -
 Cancer Free Jim Ross Confirmed For All In Wrestling Event In Texas
Jul 07, 2025
Cancer Free Jim Ross Confirmed For All In Wrestling Event In Texas
Jul 07, 2025 -
 Wrestling News Jim Ross All In 2025 Commentary Role Announced
Jul 07, 2025
Wrestling News Jim Ross All In 2025 Commentary Role Announced
Jul 07, 2025
