Limited FDA Approval: Understanding The Novavax COVID-19 Vaccine Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Limited FDA Approval: Understanding the Novavax COVID-19 Vaccine Restrictions
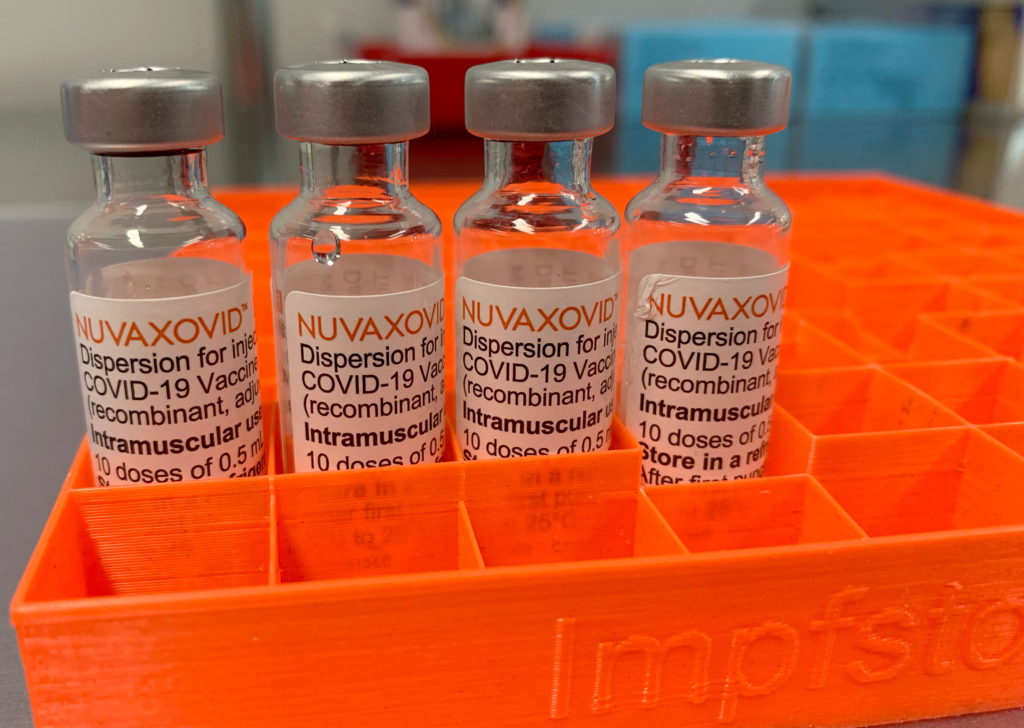
The Novavax COVID-19 vaccine, marketed as Nuvaxovid, has received limited FDA approval, sparking questions and concerns among the public. While offering a different technology compared to mRNA vaccines, its restricted authorization highlights crucial factors impacting vaccine rollout and accessibility. This article delves into the specifics of the FDA's approval, the limitations imposed, and what this means for individuals considering this vaccine option.
What is the Novavax COVID-19 Vaccine?
Nuvaxovid is a protein subunit vaccine, a technology that's been used for decades in other vaccines like the Hepatitis B vaccine. Unlike mRNA vaccines like Pfizer-BioNTech and Moderna, which utilize mRNA to instruct cells to produce viral proteins, Novavax uses lab-grown viral proteins to trigger an immune response. This difference has led some to see it as a potentially appealing alternative for individuals hesitant about mRNA technology. However, its approval pathway and limitations are crucial to understand.
The FDA's Conditional Approval and its Implications
The FDA granted Novavax emergency use authorization (EUA) initially, then later granted a limited licensure. This signifies that while deemed safe and effective, the approval comes with certain restrictions. Unlike full licensure, which often follows extensive long-term trials, this limited approval reflects the ongoing data collection and monitoring of the vaccine's long-term effects. This approach is common during public health emergencies.
Key Restrictions of the Novavax Vaccine Approval:
- Age Restrictions: The initial FDA approval was initially limited to specific age groups. While the exact age ranges might vary over time depending on further clinical data, always check the latest FDA guidelines for the most up-to-date information.
- Dosage Limitations: The authorized dosage may differ from what’s considered standard for other vaccines, again influenced by the ongoing data analysis.
- Specific Indications: The FDA might only approve the Novavax vaccine for specific populations, such as adults or individuals with underlying health conditions, excluding others.
- Limited Data: The full range of long-term side effects and efficacy across various demographics might not be fully established, leading to cautious approval and limitations.
Why the Limited Approval?
Several factors contribute to the limited FDA approval:
- Slower Development: Novavax's development timeline was slower compared to mRNA vaccines, resulting in a later entry into the market and less extensive long-term data.
- Clinical Trial Data: While the clinical trial data demonstrated efficacy and safety, it might not be as comprehensive as data collected for other vaccines that have been available longer.
- Manufacturing Challenges: Production challenges and scaling up vaccine manufacturing may have influenced the FDA's cautious approach to full licensure.
What Does this Mean for You?
The limited FDA approval of the Novavax vaccine doesn't necessarily mean it's less safe or effective. However, it's crucial to understand that the ongoing monitoring and data collection mean the approval is conditional and could change. Before making a decision, consult your healthcare provider to discuss your individual risk factors, medical history, and suitability for the Novavax vaccine compared to other available options.
Staying Informed:
For the most up-to-date information on the Novavax COVID-19 vaccine, including any changes in approval status, dosage, or eligibility, always refer to the official websites of the FDA [link to FDA website] and the CDC [link to CDC website]. Your doctor remains your best resource for personalized advice on vaccination.
Disclaimer: This article provides general information and should not be considered medical advice. Consult with a healthcare professional before making any decisions related to your health or vaccination.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Limited FDA Approval: Understanding The Novavax COVID-19 Vaccine Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Watch Now A Powerful Wwi Film Featuring Daniel Craig Cillian Murphy And Tom Hardy
May 20, 2025
Watch Now A Powerful Wwi Film Featuring Daniel Craig Cillian Murphy And Tom Hardy
May 20, 2025 -
 Tom Aspinall Responds Fan Fury Over Jon Jones Strip The Duck Comment
May 20, 2025
Tom Aspinall Responds Fan Fury Over Jon Jones Strip The Duck Comment
May 20, 2025 -
 2025s Biggest Solar Flare Widespread Radio Blackouts Reported
May 20, 2025
2025s Biggest Solar Flare Widespread Radio Blackouts Reported
May 20, 2025 -
 Spains Regulatory Crackdown Airbnb Removes Thousands Of Holiday Listings
May 20, 2025
Spains Regulatory Crackdown Airbnb Removes Thousands Of Holiday Listings
May 20, 2025 -
 Siriannis Super Bowl Success Earns Him Eagles Contract Extension
May 20, 2025
Siriannis Super Bowl Success Earns Him Eagles Contract Extension
May 20, 2025
