Limited FDA Approval: Novavax COVID-19 Vaccine's Restricted Use
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
Limited FDA Approval: Novavax COVID-19 Vaccine's Restricted Use Casts Shadow on Future
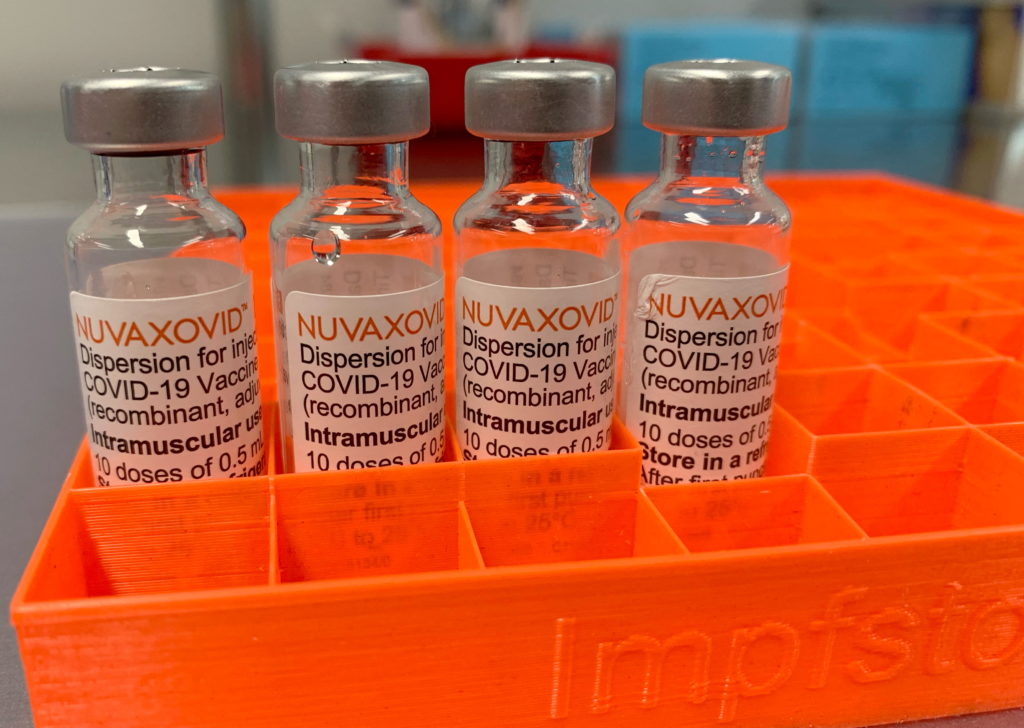
The Novavax COVID-19 vaccine, Nuvaxovid, has received limited approval from the FDA, a decision that has sparked debate and uncertainty within the medical community and public health sector. While hailed as a potential alternative for those hesitant about mRNA vaccines, its restricted use highlights challenges in vaccine development and deployment during a pandemic's waning stages. This article delves into the reasons behind the limited approval, the implications for public health, and the future prospects of the Novavax vaccine.
Why the Limited Approval?
The FDA's approval is limited primarily due to several factors. Firstly, the efficacy data, while showing effectiveness against severe illness, hospitalization, and death, lagged behind the mRNA vaccines. Secondly, the overall demand for COVID-19 vaccines has significantly decreased since the initial surge of the pandemic, reducing the urgency for widespread adoption of a new vaccine. Finally, concerns surrounding potential adverse effects, though not necessarily more prevalent than other vaccines, contributed to the cautious approach by the FDA. These factors collectively led to a more conservative rollout strategy compared to the earlier, widespread deployment of Pfizer-BioNTech and Moderna vaccines.
Implications for Public Health
The limited approval of the Novavax vaccine raises several critical questions regarding its impact on public health:
- Reaching Vaccine Hesitant Populations: Novavax's protein-based technology differs significantly from the mRNA vaccines, potentially appealing to individuals hesitant about mRNA technology. However, the limited availability might hinder efforts to reach these populations effectively.
- Vaccine Equity: The restricted use could exacerbate existing inequalities in vaccine access, particularly in underserved communities. Limited distribution may further marginalize populations already facing barriers to healthcare.
- Future Pandemic Preparedness: The experience with the Novavax vaccine highlights the challenges of developing and deploying new vaccines rapidly during a pandemic. Lessons learned from this experience are crucial for improving pandemic preparedness strategies in the future.
The Future of Nuvaxovid
The long-term prospects of Nuvaxovid remain uncertain. The current limited approval might hamper its widespread adoption, making it difficult to recoup the significant investment in research and development. However, the vaccine could still play a role in specific contexts, such as:
- Specific Populations: The vaccine may find utility in certain populations where mRNA vaccines are contraindicated or less effective.
- Booster Campaigns: Depending on future variant emergence and waning immunity, Nuvaxovid could find a niche in booster campaigns.
- Global Health Initiatives: The vaccine's potential role in global vaccination efforts remains a possibility, particularly in regions with limited access to mRNA vaccines.
Conclusion:
The FDA's decision to grant limited approval for the Novavax COVID-19 vaccine underscores the complexities of vaccine development and deployment. While offering a potential alternative for some, its restricted use highlights the need for careful consideration of efficacy data, public health needs, and resource allocation during a pandemic. The future success of Nuvaxovid will hinge on its ability to address specific needs and overcome the challenges posed by the current landscape of COVID-19 vaccination. Further research and monitoring of its efficacy and safety profile will be crucial in determining its long-term role in combating COVID-19 and preparing for future outbreaks. For more information on COVID-19 vaccines, visit the .

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Limited FDA Approval: Novavax COVID-19 Vaccine's Restricted Use. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Helldivers 2 Masters Of Ceremony Warbond Drop Details For May 15th
May 20, 2025
Helldivers 2 Masters Of Ceremony Warbond Drop Details For May 15th
May 20, 2025 -
 International Support Needed Bali Prioritizes Tourist Safety And Conduct
May 20, 2025
International Support Needed Bali Prioritizes Tourist Safety And Conduct
May 20, 2025 -
 Jenn Sterger Reflects On The Brett Favre Scandal A Story Of Betrayal And Disrespect
May 20, 2025
Jenn Sterger Reflects On The Brett Favre Scandal A Story Of Betrayal And Disrespect
May 20, 2025 -
 Jamie Lee Curtis Reveals The Status Of Her Bond With Lindsay Lohan Post Freaky Friday
May 20, 2025
Jamie Lee Curtis Reveals The Status Of Her Bond With Lindsay Lohan Post Freaky Friday
May 20, 2025 -
 Strip The Duck Jon Jones Aspinall Comments Spark Heated Debate Among Mma Fans
May 20, 2025
Strip The Duck Jon Jones Aspinall Comments Spark Heated Debate Among Mma Fans
May 20, 2025
