Limited FDA Approval: Novavax COVID-19 Vaccine's Conditional Authorization
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
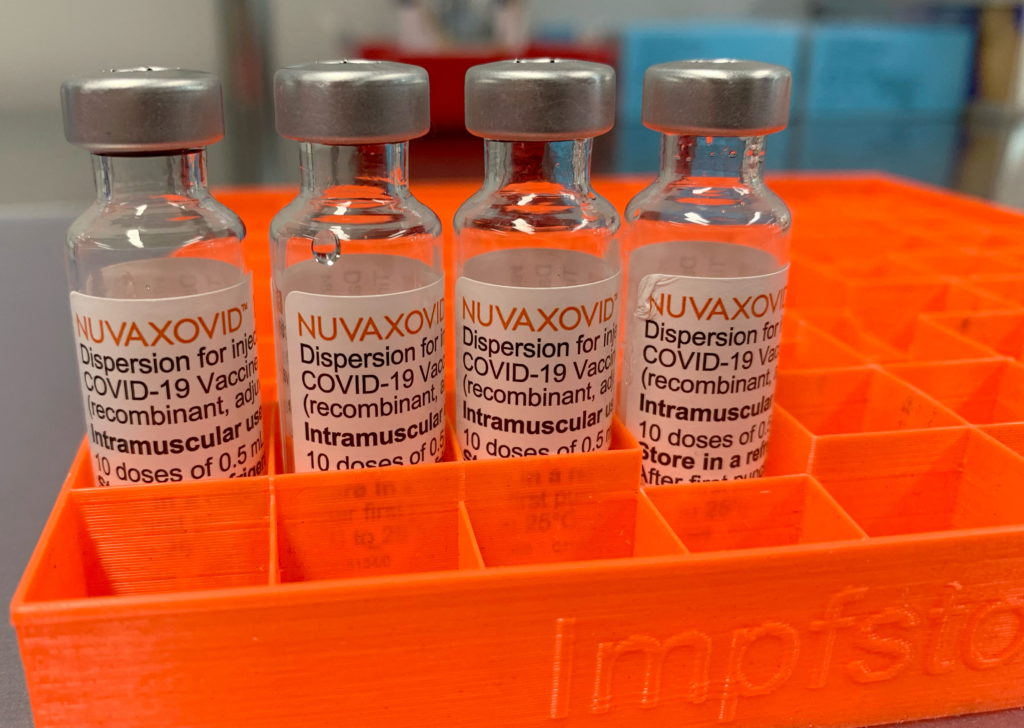
Limited FDA Approval: Novavax COVID-19 Vaccine's Conditional Authorization Raises Questions
The Novavax COVID-19 vaccine, Nuvaxovid, has received limited approval from the FDA, marking a significant yet cautious step in the ongoing fight against the pandemic. This conditional authorization, however, raises several questions about its role in the current vaccination landscape, especially considering the widespread availability of other vaccines. Let's delve into the details.
What is the FDA's Conditional Authorization?
The Food and Drug Administration (FDA) granted Nuvaxovid a conditional authorization for individuals 18 years of age and older. This isn't a full approval; it's a pathway that allows for the use of a vaccine while further data is collected. This conditional nature reflects the ongoing need for post-market surveillance to fully assess the long-term safety and efficacy of the vaccine. Unlike the full approvals granted to other COVID-19 vaccines, this limited authorization comes with a stricter monitoring process. The FDA will continue to evaluate the data gathered to confirm the vaccine's ongoing safety and effectiveness.
Why is this Significant (and Why Not)?
The arrival of a new COVID-19 vaccine is undeniably significant. Novavax utilizes a protein subunit technology, a different approach than the mRNA vaccines from Pfizer-BioNTech and Moderna, or the viral vector vaccines like Johnson & Johnson's. This offers an alternative for individuals who may have concerns about mRNA or viral vector technology. However, its significance is tempered by the fact that many are already vaccinated, and booster uptake, while variable, is also significant. The need for a new vaccine is less pressing than it was in the earlier stages of the pandemic.
Potential Advantages of the Novavax Vaccine:
- Different Technology: As mentioned above, its protein subunit technology might appeal to those hesitant about mRNA or viral vector vaccines.
- Potential for Wider Acceptance: This could lead to increased vaccination rates among hesitant populations.
- Contribution to Herd Immunity: Increased vaccination rates, even with a limited rollout, could still contribute to a stronger herd immunity within the population.
Concerns and Challenges:
- Limited Demand: Given the existing vaccination rates and availability of other vaccines, demand for Novavax's vaccine might be limited.
- Conditional Authorization: This highlights the ongoing need for further safety and efficacy monitoring.
- Competition in the Market: The vaccine faces strong competition from already established and widely accepted COVID-19 vaccines.
- Efficacy Concerns: While initial trial data showed efficacy, long-term real-world data is still needed to definitively assess its effectiveness against circulating variants.
What This Means for the Future of COVID-19 Vaccination:
The conditional approval of the Novavax COVID-19 vaccine presents a complex scenario. While it offers an alternative technology and potentially increases vaccination rates, its impact will depend on several factors, including public perception, healthcare provider recommendations, and the evolving epidemiological landscape. The FDA's continued monitoring and data collection will be crucial in determining the long-term role of Nuvaxovid in COVID-19 vaccination strategies. Further research will also be critical in understanding its effectiveness against emerging variants of concern.
Further Reading:
For more detailed information, refer to the official FDA website [link to FDA website]. You can also explore the CDC’s resources on COVID-19 vaccination [link to CDC website].
Call to Action: Stay informed about the latest updates on COVID-19 vaccines and consult with your healthcare provider to make informed decisions about your vaccination strategy.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on Limited FDA Approval: Novavax COVID-19 Vaccine's Conditional Authorization. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 World Peace Appeal Key Moments From Pope Leos Inauguration Ceremony
May 20, 2025
World Peace Appeal Key Moments From Pope Leos Inauguration Ceremony
May 20, 2025 -
 Ukraine War Update Trump Announces Plans For Immediate Peace Talks
May 20, 2025
Ukraine War Update Trump Announces Plans For Immediate Peace Talks
May 20, 2025 -
 Overcompensating Features One Of 2024s Most Unexpectedly Funny Sex Scenes
May 20, 2025
Overcompensating Features One Of 2024s Most Unexpectedly Funny Sex Scenes
May 20, 2025 -
 Freaky Friday Reunion Jamie Lee Curtis Talks Post Film Relationship With Lindsay Lohan Exclusive
May 20, 2025
Freaky Friday Reunion Jamie Lee Curtis Talks Post Film Relationship With Lindsay Lohan Exclusive
May 20, 2025 -
 Exclusive Interview The Making Of Netflixs Fall Of Favre And Its Controversial Subject
May 20, 2025
Exclusive Interview The Making Of Netflixs Fall Of Favre And Its Controversial Subject
May 20, 2025
