FDA Greenlights Novavax COVID-19 Vaccine With Unusual Usage Restrictions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
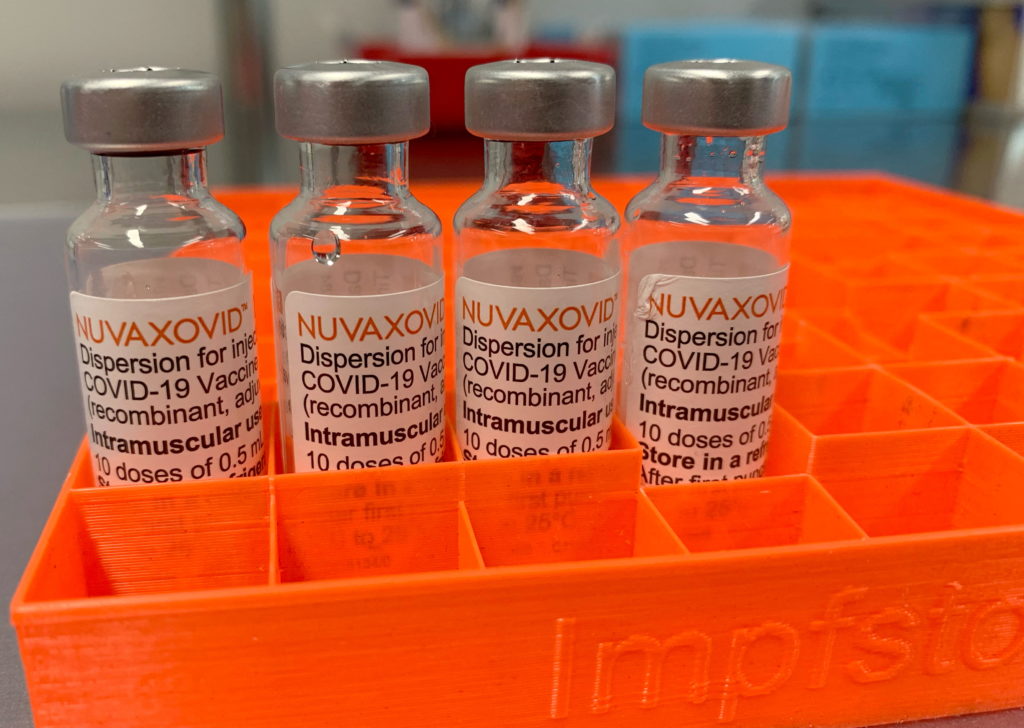
FDA Greenlights Novavax COVID-19 Vaccine, But with Unusual Usage Restrictions
The FDA's approval of the Novavax COVID-19 vaccine, Nuvaxovid, has been met with a mix of relief and cautious optimism. While hailed as a much-needed addition to the arsenal against the virus, the agency's decision comes with some unusual and noteworthy usage restrictions, prompting questions and raising eyebrows within the medical community.
A New Contender in the COVID-19 Vaccine Race
Novavax's protein-based vaccine represents a different approach compared to the mRNA vaccines from Pfizer-BioNTech and Moderna, or the viral vector vaccine from Johnson & Johnson. This protein subunit technology has been used for decades in other vaccines, potentially appealing to individuals hesitant about mRNA technology. This difference in technology is a key element that might influence vaccine uptake, especially among those who have previously expressed concerns about mRNA vaccines. The FDA's approval, therefore, was anticipated by many as a significant development in the fight against COVID-19.
The Unexpected Restrictions: A Closer Look
However, the FDA's approval isn't unqualified. The agency has placed significant usage restrictions on Nuvaxovid, primarily focusing on its authorization only for individuals 18 years of age and older. This is noteworthy because other authorized COVID-19 vaccines have broader age ranges. Furthermore, the FDA has emphasized the limited data available on the vaccine's effectiveness against newer variants of the virus, including Omicron subvariants. This points to the need for ongoing surveillance and further research to ascertain its long-term efficacy and safety profile against emerging variants.
Why the Restricted Authorization?
The FDA's justification for the restricted authorization hinges on the comparatively smaller clinical trial data available for Novavax's vaccine compared to its competitors. While the trials demonstrated efficacy and safety within the approved age range, the limited data concerning younger populations and evolving virus strains necessitates a more cautious approach. This highlights the stringent regulatory processes the FDA undertakes to ensure the safety and efficacy of any vaccine before widespread deployment.
What Does This Mean for the Future of COVID-19 Vaccination?
The approval of Nuvaxovid, despite the restrictions, still offers a valuable option for individuals who prefer a protein-based vaccine or who may have had concerns about mRNA vaccines. However, the limited age range and the caveats regarding variant effectiveness underscore the dynamic nature of the COVID-19 pandemic and the ongoing need for research and development in the field of vaccine technology. The FDA’s decision underscores the importance of a multi-pronged approach to vaccination, using different vaccine platforms to combat the virus effectively.
Looking Ahead:
The ongoing monitoring of Nuvaxovid's performance and the collection of further data are crucial. Future studies could potentially expand the authorized age range and offer a clearer picture of its effectiveness against emerging variants. The situation highlights the need for continued vigilance and adaptive strategies in the face of evolving viral mutations. For the latest updates on COVID-19 vaccines and recommendations, consult the .
Keywords: Novavax, COVID-19 vaccine, FDA approval, Nuvaxovid, protein-based vaccine, vaccine restrictions, mRNA vaccine, vaccine efficacy, COVID-19 variants, Omicron, vaccine safety, public health, vaccination strategy.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Greenlights Novavax COVID-19 Vaccine With Unusual Usage Restrictions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 Tornado Watch Lifted In North Texas Dallas Weather Report
May 20, 2025
Tornado Watch Lifted In North Texas Dallas Weather Report
May 20, 2025 -
 Jamie Lee Curtis Reveals The Truth About Her Friendship With Lindsay Lohan
May 20, 2025
Jamie Lee Curtis Reveals The Truth About Her Friendship With Lindsay Lohan
May 20, 2025 -
 Us Treasury Yield Decline Follows Feds 2025 Rate Cut Prediction
May 20, 2025
Us Treasury Yield Decline Follows Feds 2025 Rate Cut Prediction
May 20, 2025 -
 Severe Solar Storm Triggers Continent Wide Communications Blackouts
May 20, 2025
Severe Solar Storm Triggers Continent Wide Communications Blackouts
May 20, 2025 -
 American Innovation How Medical And Scientific Research Drives National Strength
May 20, 2025
American Innovation How Medical And Scientific Research Drives National Strength
May 20, 2025
