FDA Greenlights Novavax COVID-19 Vaccine, But With Strict Usage Conditions
Welcome to your ultimate source for breaking news, trending updates, and in-depth stories from around the world. Whether it's politics, technology, entertainment, sports, or lifestyle, we bring you real-time updates that keep you informed and ahead of the curve.
Our team works tirelessly to ensure you never miss a moment. From the latest developments in global events to the most talked-about topics on social media, our news platform is designed to deliver accurate and timely information, all in one place.
Stay in the know and join thousands of readers who trust us for reliable, up-to-date content. Explore our expertly curated articles and dive deeper into the stories that matter to you. Visit Best Website now and be part of the conversation. Don't miss out on the headlines that shape our world!
Table of Contents
FDA Greenlights Novavax COVID-19 Vaccine, but with Strict Usage Conditions
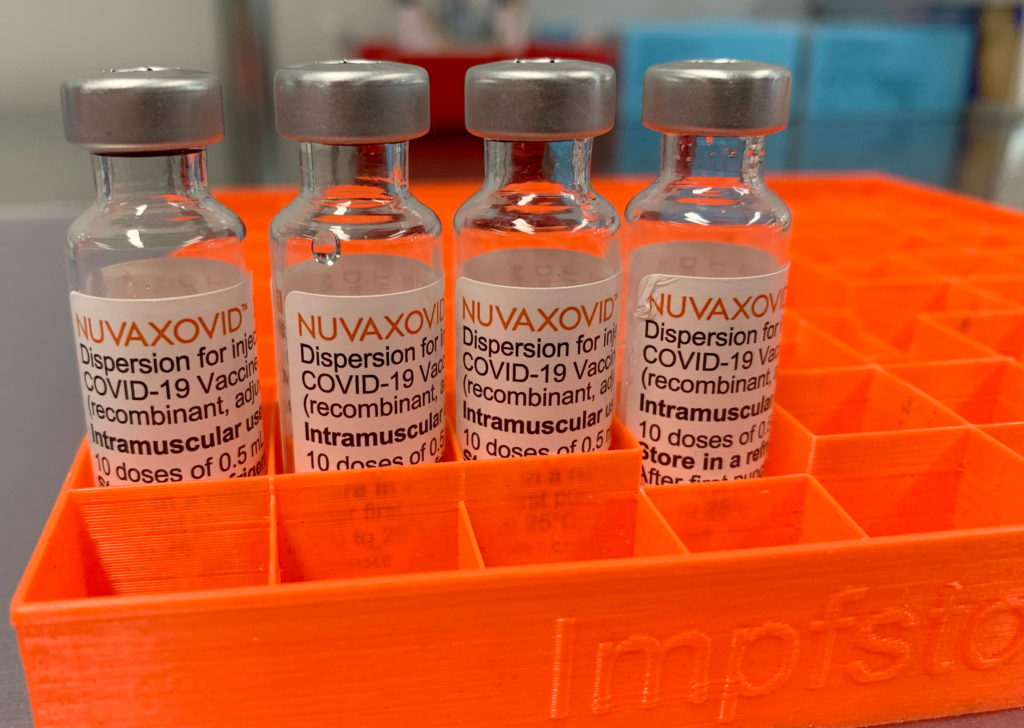
The FDA has finally granted Emergency Use Authorization (EUA) to the Novavax COVID-19 vaccine, Nuvaxovid, offering a new option for those seeking vaccination. However, the authorization comes with strict usage conditions, limiting its immediate impact. This marks a significant development in the ongoing fight against the pandemic, but the nuances of the approval warrant a closer look.
A Different Approach: Protein Subunit Technology
Unlike the mRNA vaccines from Pfizer-BioNTech and Moderna, or the viral vector vaccine from Johnson & Johnson, Novavax utilizes a protein subunit technology. This approach uses lab-grown pieces of the virus to trigger an immune response, avoiding the use of mRNA or live viruses. This difference may appeal to some individuals hesitant about the other vaccine technologies. This makes it a valuable addition to the existing COVID-19 vaccine arsenal, offering an alternative for those who may have concerns about mRNA vaccines. For more information on different vaccine technologies, you can check out resources from the .
Strict Usage Conditions: Why the Limited Rollout?
While the approval is a victory for Novavax, the FDA's EUA comes with significant limitations. These conditions include:
- Limited Age Range: The vaccine is currently authorized only for individuals 18 years of age and older. Trials for younger age groups are still underway.
- Specific Dosage: The vaccine requires a two-dose primary series, administered three weeks apart.
- Potential Side Effects: Like all vaccines, Novavax can cause side effects. Common side effects include pain at the injection site, fatigue, headache, muscle aches, and joint pain. Severe allergic reactions, while rare, are possible. Individuals with a history of severe allergic reactions should consult their physician before receiving the vaccine.
- Efficacy Concerns: While effective in clinical trials, the vaccine's efficacy may be slightly lower compared to some mRNA vaccines, particularly against certain variants. The FDA continues to monitor its effectiveness against emerging variants.
The Future of Novavax: Potential and Challenges
The approval of the Novavax vaccine represents a potential turning point in global vaccination efforts. Its protein subunit technology could offer an attractive alternative for individuals hesitant about mRNA vaccines, potentially increasing vaccine uptake worldwide. However, the strict usage conditions and the need for further research on efficacy against evolving variants pose significant challenges to its widespread adoption.
Addressing Vaccine Hesitancy:
The availability of the Novavax vaccine could play a critical role in addressing vaccine hesitancy. Providing a diverse range of vaccine options allows individuals to choose a vaccine that best suits their individual needs and concerns. Open and honest communication about the benefits and risks of each vaccine is crucial in building public trust and encouraging vaccination.
What this Means for You:
The FDA's authorization of the Novavax vaccine expands the available options for COVID-19 prevention. However, it’s important to discuss your individual health needs and vaccination choices with your healthcare provider to determine the most appropriate course of action. Stay informed about the latest updates from reliable sources like the CDC and FDA. The fight against COVID-19 is ongoing, and informed decisions are essential.
Keywords: Novavax, COVID-19 vaccine, FDA approval, EUA, protein subunit vaccine, vaccine hesitancy, vaccine safety, Nuvaxovid, COVID-19 vaccination, vaccine technology, mRNA vaccine, viral vector vaccine.

Thank you for visiting our website, your trusted source for the latest updates and in-depth coverage on FDA Greenlights Novavax COVID-19 Vaccine, But With Strict Usage Conditions. We're committed to keeping you informed with timely and accurate information to meet your curiosity and needs.
If you have any questions, suggestions, or feedback, we'd love to hear from you. Your insights are valuable to us and help us improve to serve you better. Feel free to reach out through our contact page.
Don't forget to bookmark our website and check back regularly for the latest headlines and trending topics. See you next time, and thank you for being part of our growing community!
Featured Posts
-
 The Making Of Untold Brett Favre A J Perez On Facing Pressure And Production Issues
May 21, 2025
The Making Of Untold Brett Favre A J Perez On Facing Pressure And Production Issues
May 21, 2025 -
 New Streaming Release A Gripping Wwi Film Starring Daniel Craig Cillian Murphy And Tom Hardy
May 21, 2025
New Streaming Release A Gripping Wwi Film Starring Daniel Craig Cillian Murphy And Tom Hardy
May 21, 2025 -
 Corporate Value And Nature Conservation New Guidelines For 160 Japanese Companies In 13 Industries
May 21, 2025
Corporate Value And Nature Conservation New Guidelines For 160 Japanese Companies In 13 Industries
May 21, 2025 -
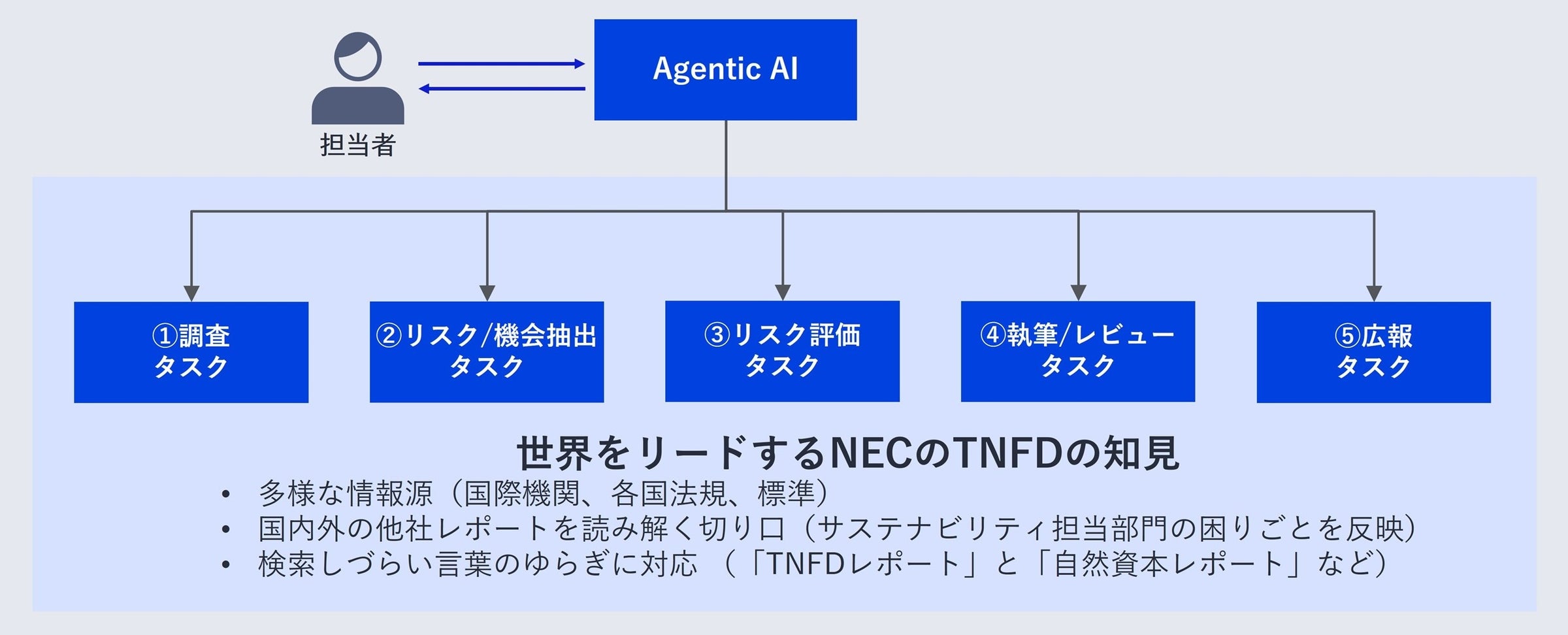 Nec Ai
May 21, 2025
Nec Ai
May 21, 2025 -
 Bidens Cancer Diagnosis A Wave Of Support From World Leaders
May 21, 2025
Bidens Cancer Diagnosis A Wave Of Support From World Leaders
May 21, 2025
